Factors that Affect Rate of Reaction
Factors that Affect Rate of Reaction Revision
Factors that Affect Rate of Reaction
Rates of reaction tell us how fast or slow a given reaction is. The rate of a chemical reaction is heavily influenced by a number of factors. The chief conditions that affect rate of reaction are; temperature, pressure, and concentration. Two other important factors are the surface area of the reactants and the presence of a catalyst. The effects of these factors can be explained by considering chemical reactions in terms of collision theory.
Collision Theory and Activation Energy
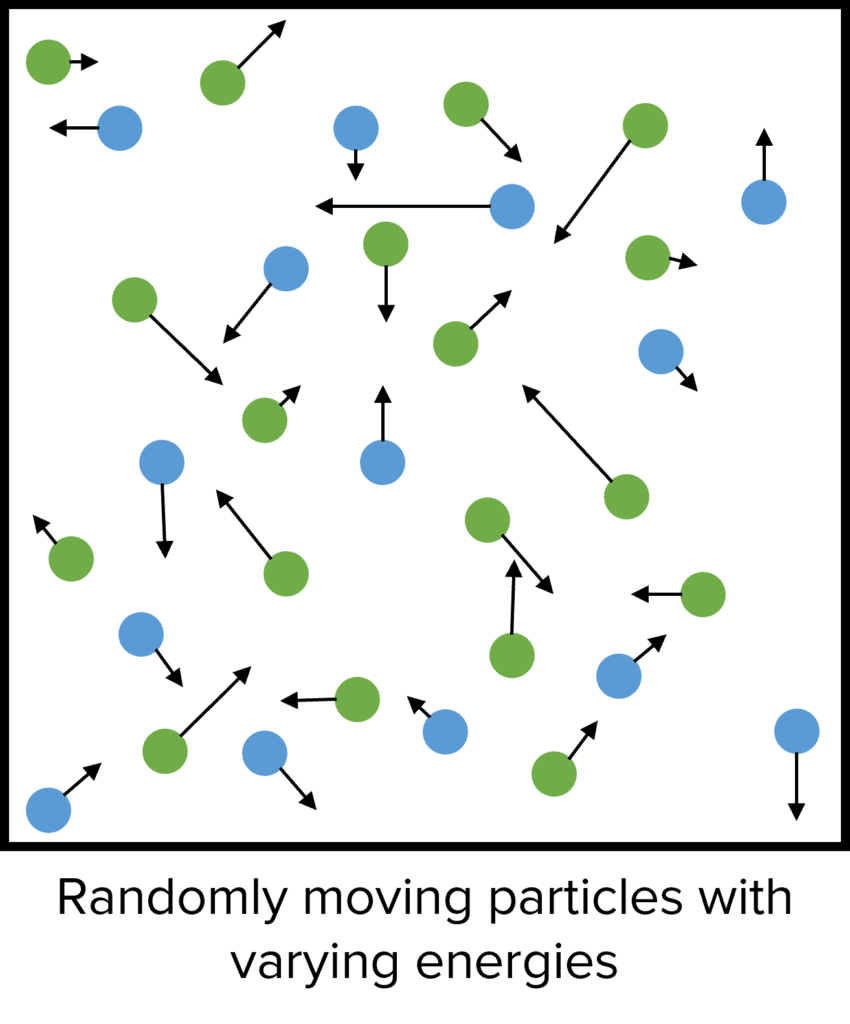
When particles undergo chemical reactions, they move around randomly in the reaction mixture with lots of different energies. These randomly moving particles will often collide with each other and, if the right conditions are met, they will react with one another. This model of chemical reactions is known as collision theory.
In collision theory, collisions between particles that lead to a reaction are referred to as successful collisions. The more successful collisions there are in a reaction, the higher the rate of reaction will be. As such, to increase rate of reaction, it is desirable to do anything that will increase the frequency of successful collisions between particles.
For a collision to be successful, the particles must collide with enough energy. The minimum amount of energy required for a collision to be successful is know as the activation energy of the reaction. The activation energy is the energy needed to break the bonds of the reactants so that products can be formed.
The requirement for particles to have at least the activation energy for a collision to be successful creates two scenarios.
- Particles in the reaction collide at high speed. They have energy that is at least equal to the activation energy of the reaction. The bonds in these particles are broken and new ones formed to produce reaction products. This is a successful collision.
- Particles in the reaction mixture collide at slower speed. They do not have enough energy to meet the activation energy of the reaction. They do not react and so simply bounce off one another. This is an unsuccessful collision.
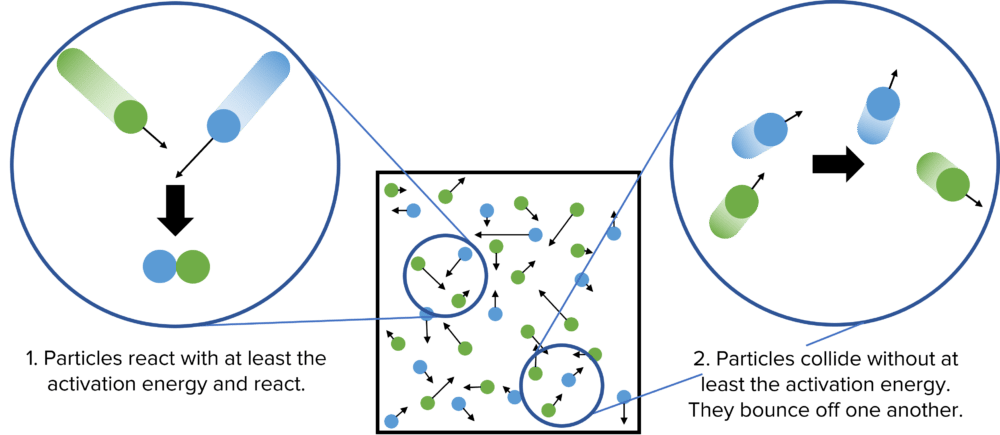
The Effects of Different Conditions on Rate of Reaction
The rate of a chemical reaction can be increased by increasing the number of successful collisions happening in a given time. This is called the frequency of successful collisions. Anything that increases the frequency of successful collisions will increase the rate of reaction. There are a number of different factors that will affect this frequency, including temperature, pressure, and concentration.
1. Temperature
Increasing the temperature of a reaction will increase the energy of the particles with in it. This in turn increases the proportion of particles colliding with at least enough energy to meet the activation energy of reaction. Therefore, by increasing the energy of the particles the frequency of successful collisions is increased.
Conversely, reducing the temperature of a reaction will decrease the energy of the particles, lowering the frequency of successful collisions and hence lowering the rate of reaction.
2. Pressure
Increasing the pressure under which a reaction is taking place will lead to an increase in the frequency of successful collisions. Consider a container containing two reacting gasses. The particles of gas are able to move around inside the container, colliding with each other as well as the walls of the container. A set number proportion – let us say 10\% – of these collisions are successful and lead to a reaction.
If we then reduce the size of the container, the same number of particles will have less space to move about in. This will increase the pressure under which these gasses are reacting. This will increase the total number of collisions between particles.
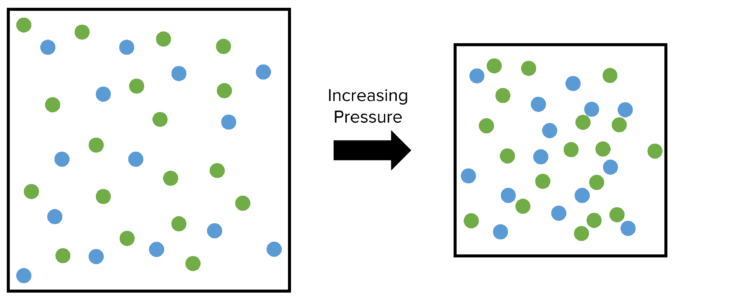
However, the energy of the particles remains unchanged. As such the proportion of these collisions that are successful remains the same \left(10\%\right). The number of successful collisions increases because the total number of collisions increases.
3. Concentration
Increasing the concentration of reactants will also increase the total number of collisions. In this case, imagine a container containing reacting gasses. Instead of reducing the size of the container, we double the concentrations of the reacting gasses. This will double the number of particles present in the container.
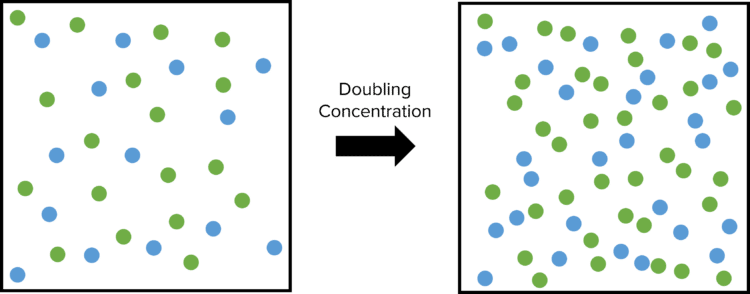
This will reduce the space that each particle has to move around in and will lead to an increase in the total number of collisions. Therefore, increasing the concentration of reactants will increase the frequency of successful collisions.
Surface Area
Most chemical reactions take place in the liquid or solution phases, where all the particles of the reactants are able to move around and collide. However, in some reactions (such as the thermal decomposition of calcium carbonate) one or more of the reactions is in the solid phase. In these cases, the majority of the particles of the solid reactants are found in the centre of the solid. These particles are unable to react as no particles of the other reactants can collide with them.
The only particles of a solid reactant that can react are those that are found at the surface of the solid.
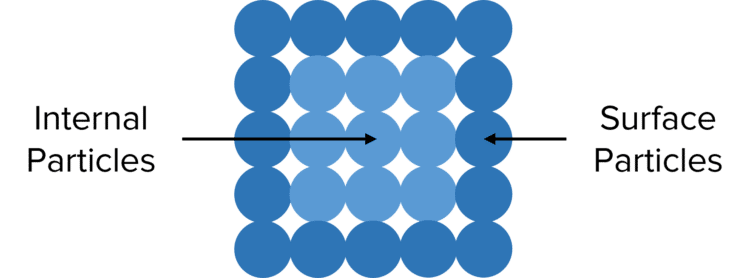
In reactions where one or more reactants exists as a large single solid, the rate of reaction will be relatively low as the number of surface particles able to take part in successful collisions is quite low.
To increase the rate of reaction, we need to increase the number of particles at the surface of the of the solid. To do this we need to increase the surface area of the solid. This can be done by reducing the size of the pieces of a solid by crushing them up into powders.
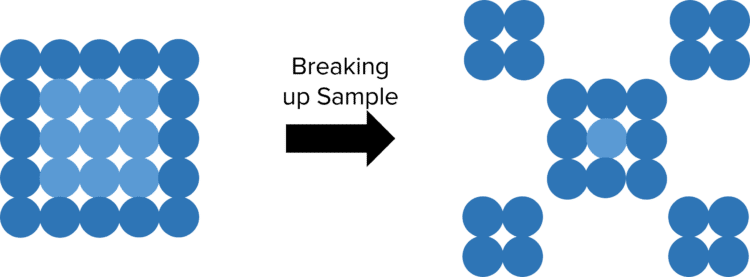
By crushing the solid up into a powdered form, we increase the total surface area of the reactant, and as such drastically increases the amount of particles at the surface. This allows for a greater number of collisions between reactant particles, leading to more frequent successful collisions and so a higher rate of reaction.
This relationship can also be described in terms of the surface area to volume ratio of a solid. The surface area to volume ratio of a solid will tell us about how much of the solid is contained at the surface of the solid compared to the centre. The ratio is calculated by dividing the surface area of a solid by its volume:
\text{Surface Area-Volume Ratio}=\frac{\text{Surface Area}}{\text{Volume}}
When we powder a solid, we increase the value of its surface area to volume ratio. A higher ratio will lead to a faster rate of reaction.
Catalysis
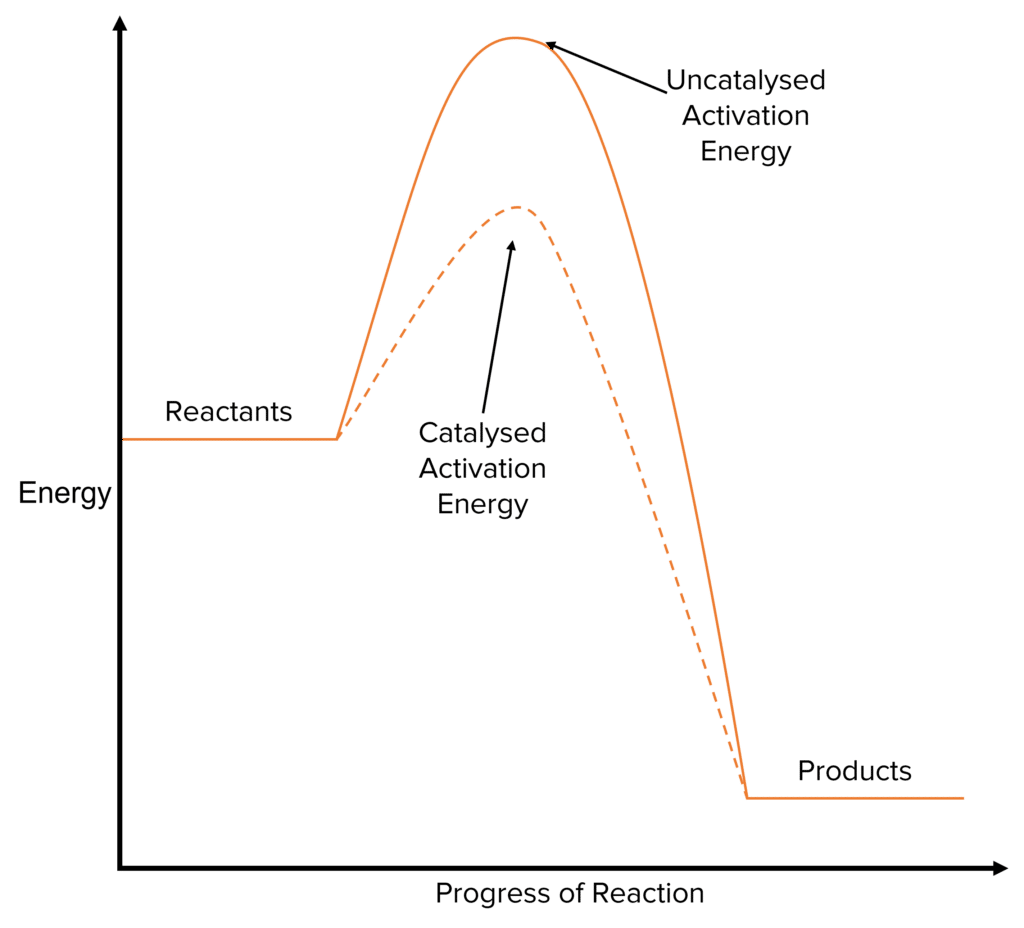
A catalyst is a substance that takes part in a chemical reaction without itself being chemically altered at the end of the reaction. This means that, though a catalyst may interact with the reactants in a chemical reaction, it is not used up and is typically regenerated in the reaction. For this reason, catalysts are not included in the overall chemical equation of a reaction.
Catalysts are added to speed up chemical reactions. They do this by providing an alternative reaction pathway with a lower activation energy than the uncatalysed reaction. This will lead to more particles in the system having at least the minimum energy needed to react. This increases the proportion of collisions in the reaction that are successful, leading to a higher rate of reaction.
Catalysts are commonly used throughout the chemical industry to speed up chemical reactions, that would otherwise be to slow to use. There are also many examples of catalysis in nature. One of the most important of these examples is that of enzymes. Enzymes are biological catalysts that aid in many of the body’s functions.
Required Practical
Investigating Rates of Reaction.
A number of different experiments can be carried out to determine the effects of changing different factors on the rate of a chemical reaction. One such experiment involves the reaction of magnesium and hydrochloric acid. In this experiment, the gas evolved by the reaction is collected and used to calculate the rate of reaction.
Another experiment involves timing how long it takes for a reaction mixture to become opaque. In this method, a black coloured X is placed below a beaker containing chemicals that, upon reaction, produce an opaque solution. This obscures the X, allowing the progress of the reaction to be monitored.
Method 1 – Gas Collection
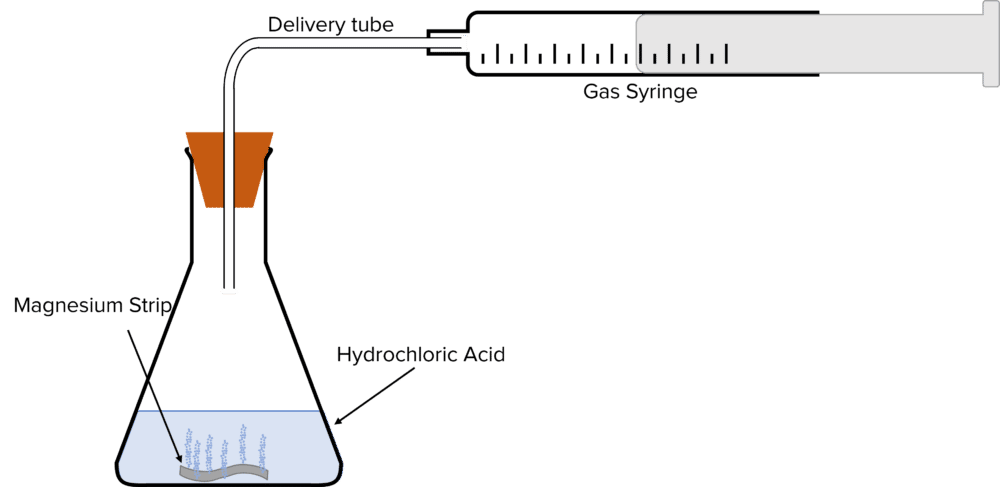
- Clean a strip of magnesium with emery paper.
- Place the strip of magnesium into a conical flask containing 25\text{ cm}^{3} of a 0.1\text{ g}/\text{cm}^{3} solution of hydrochloric acid.
- Immediately upon addition of the hydrochloric acid, seal the flask with a bung with a delivery tube inserted through the centre. Connect the delivery tube to a gas syringe.
- Once the gas syringe is attached, start a timer.
- Record the volume of gas in the syringe every 10 seconds until the volume has been constant for at least 3 readings.
- Record the time of the first reading at which the volume of gas in the syringe stopped increasing.
- Repeat steps 1 to 6, each time increasing the concentration of hydrochloric acid by 0.1\text{ g}/\text{cm}^{3} each time until the acid has reached 1\text{ g}/\text{cm}^{3}.
Method 2 – Opaque Reaction Mixtures
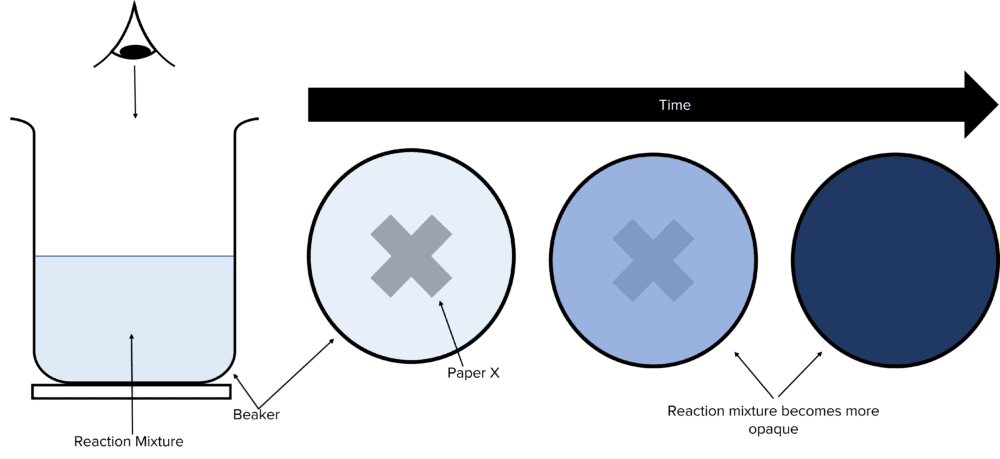
- Place a white tile with a black X painted on under a glass beaker.
- Pour 25\text{ cm}^{3} of a 0.5\text{ g}/\text{ cm}^{3} of sodium thiosulfate into the beaker.
- To this add 25\text{ cm}^{3} of a 0.1\text{ g}/\text{ cm}^{3} solution of hydrochloric acid.
- Immediately start a timer.
- Watch the black X from above the beaker. Stop the timer once the cross is no longer visible through the reaction mixture.
- Record the time on the timer.
- Repeat steps 1 to 6, each time increasing the concentration of hydrochloric acid by 0.1\text{ g}/\text{cm}^{3} each time until the acid has reached 1\text{ g}/\text{cm}^{3}.
Analysis
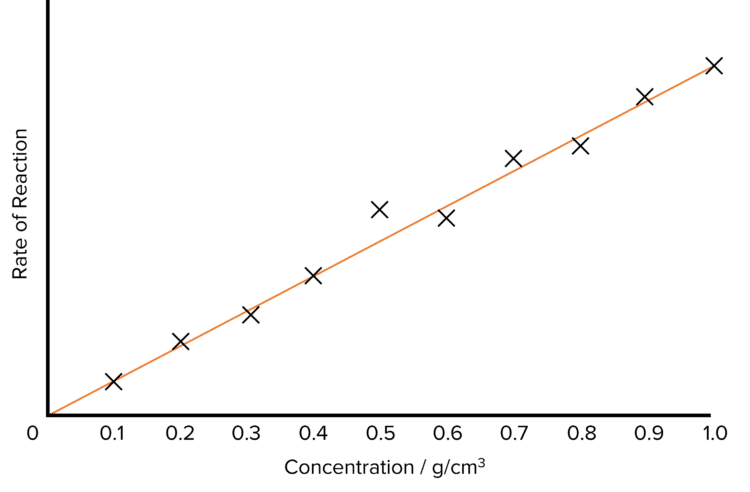
- Using the data collected, calculate the rate of reaction for each concentration of hydrochloric acid.
- Plot the rate of reaction against the concentration of acid and draw a line of best fit.
- The graph should produce a straight line of best fit with a positive gradient.
Factors that Affect Rate of Reaction Example Questions
Question 1: Predict and explain the effect of decreasing the concentration of hydrochloric acid in reaction with magnesium.
[4 marks]
- The rate of reaction will decrease.
- The number of reactant particles will decrease.
- So the total number of collisions will decrease, leading to a lower frequency of successful collisions.
Question 2: Define the term ‘activation energy’.
[2 marks]
The minimum amount of energy with which particles must collide to be able to react.
Question 5: Define the term ‘catalyst’.
[2 marks]
A substance that increases the rate of a chemical reaction while itself remaining chemically unchanged.
Question 4: Explain why flour in a bag will not ignite when touched with a lit splint but will ignite if sprayed through a flame.
[5 marks]
- In a bag of flour the flour is packed densely and has a lower surface area.
- This means means that it there are fewer flour particles available to react with oxygen in the air.
- This prevents ignition.
- When spayed through the flame the individual flour particles spread out.
- This greatly increases the surface area of the flour.
- This increases the number of flour particles able to react the flour leading to ignition.









