Reversible Reactions and Equilibria
Reversible Reactions and Equilibria Revision
Reversible Reactions and Equilibria
In many chemical reactions, the reaction will proceed only in the forward direction, from reactants to products. Some chemical reactions are however reversible. This means that they can proceed in the backward direction, from products to reactants. When the forward and backward directions of a reversible reaction are balanced an equilibrium is set up.
Reversible Reactions
Some chemical reactions are non-reversible. When we write out the equation for these reactions, we show that they can only proceed in one direction using a single arrow moving from the reactants on the right to the products on the left.
For example, in the reaction between vinegar (ethanoic acid) and sodium hydrogen carbonate, one of the products is carbon dioxide. This is produced as a gas leaves the reaction, preventing a backwards reaction:
\text{CH}_3\text{COOH}_{\text{(aq)}}+\text{NaHCO}_{3\text{(aq)}}\rarr\text{CH}_3\text{COONa}_{\text{(aq)}}+\text{H}_2\text{O}_{\text{(l)}}+\text{CO}_{2\text{(g)}}
However, in some cases, the products of a chemical reaction will be able to react with one another to regenerate the reactants. When chemical reactions are able to move in both the forward and backward directions, the reaction is referred to as being reversible.
One example of a reversible reaction is the reaction of hydrogen gas and iodine vapour to form hydrogen iodide. In the forward reaction hydrogen and iodine react to form \text{HI}. In the backwards reaction two molecules of \text{HI} react to form one molecule of hydrogen and one of iodine:
\text{Forward Reaction: } \text{H}_2+\text{I}_2\textcolor{#aa57ff}{\rarr}2\text{HI}
\text{Backward Reaction: }\text{HI}+\text{HI}\textcolor{#aa57ff}{\rarr}\text{H}_2+\text{I}_2
It would be a lot of work to write two equations every time a reversible reaction is mentioned. Instead, only the forward reaction is written. To indicate that the reaction is reversible, the single forward arrow is replaced with two parallel half arrows pointing in opposite directions.
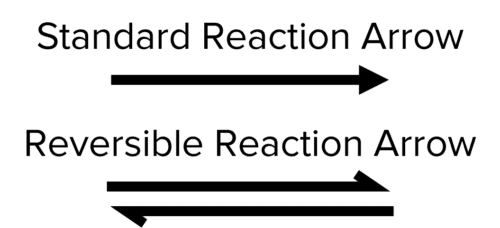
For the example above this would look like:
\text{H}_2+\text{I}_2\textcolor{#aa57ff}{\rightleftharpoons}2\text{HI}
Energy Changes in Reversible Reactions
Reversible reactions will also show opposing energy changes in the forward and backward reactions. For example the reaction to form \text{HI} is an exothermic reaction, meaning that it transfers energy to its surroundings. This is because more energy is released by the formation of the \text{H--I} bond then is absorbed in breaking the \text{H--H} and \text{I--I} bonds.
If the reverse reaction takes place, energy is absorbed, as more is needed to break the bonds in \text{HI} than is released by the formation of products. This means that the reverse reaction is endothermic.
The energy change of a reverse reaction will always be the opposite to that of the forward reaction. As such, when the forward reaction is endothermic, the backward reaction is exothermic and vice versa.
Equilibrium
When reversible reactions happen in a sealed container, none of the reactants or products can escape. This sets up an equilibrium. Equilibrium is a special case of reversible chemical reactions where the rate of the forward reaction is equal to rate of the backward reaction. In these systems, the concentrations of both reactants and products stay constant. As the rates of forward and backward reactions are equal, reactant and product are formed and used up in equal amounts.
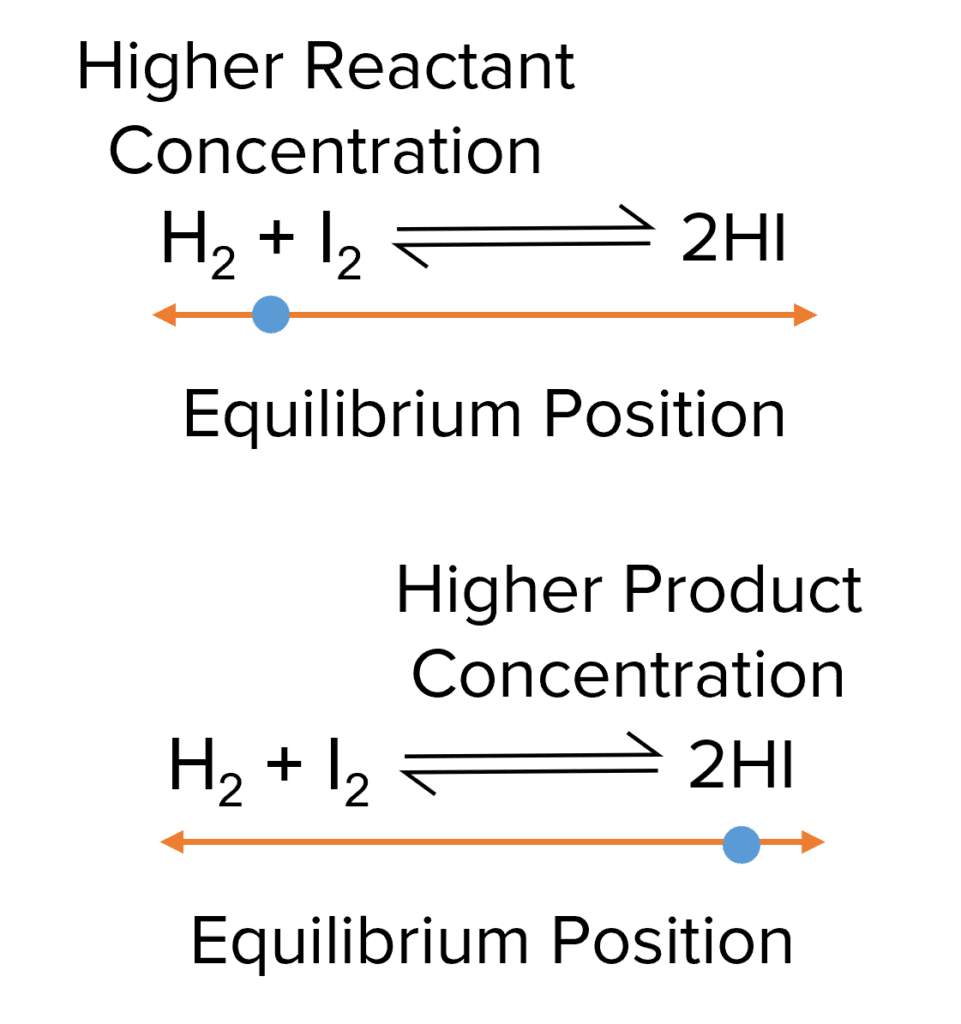
Though the concentrations of the reactants remain constant, they are not necessarily equal. In any given equilibrium, there may be a higher concentration of reactants or a higher of concentration of products. The difference in reactant and product concentrations is referred to as the position of the equilibrium.
When reactant concentration is higher are said to lie towards the reactants, or towards the left of the reaction. When the product concentration is higher, the equilibrium is said to lie towards the products, or towards the right.
The position of an equilibrium will shift in response to changes in the conditions under which the reactions are taking place. This means that the equilibrium will move towards the reactants or products of the reaction, increasing the concentration of one and decreasing the concentration of the other.
Which concentration is increased, and which is decreased will depend on how the conditions of the reaction are changed and upon the reaction itself.
The affect that changing a given condition will have an the position of an equilibrium can be predicted according to Le Chatelier’s Principle.
Le Chatelier’s Principle
Le Chatelier’s Principle is a chemical principle that allows us to predict how the position of an equilibrium will shift in response to a change in its conditions. The principle states that:
If the conditions of an equilibrium are changed, the equilibrium will shift its position in order to counteract that change.
The direction of this shift, whether it is towards the reactants or products, will be determined by which side of the equilibrium will best counteract the change imposed. Equilibria will shift in different directions according to a number of different factors, such as the moles produced on either side and the energy changes of each reaction. The three main conditions that will affect the position of an equilibrium are temperature, pressure, and concentration. We will take the formation of ammonia as an example:
\text{N}_2+3\text{H}_2\rightleftharpoons2\text{NH}_3\text{ }\Delta_\text{f}\text{H}^{\varnothing}=-46\text{ kJ mol}^{-1}
Temperature
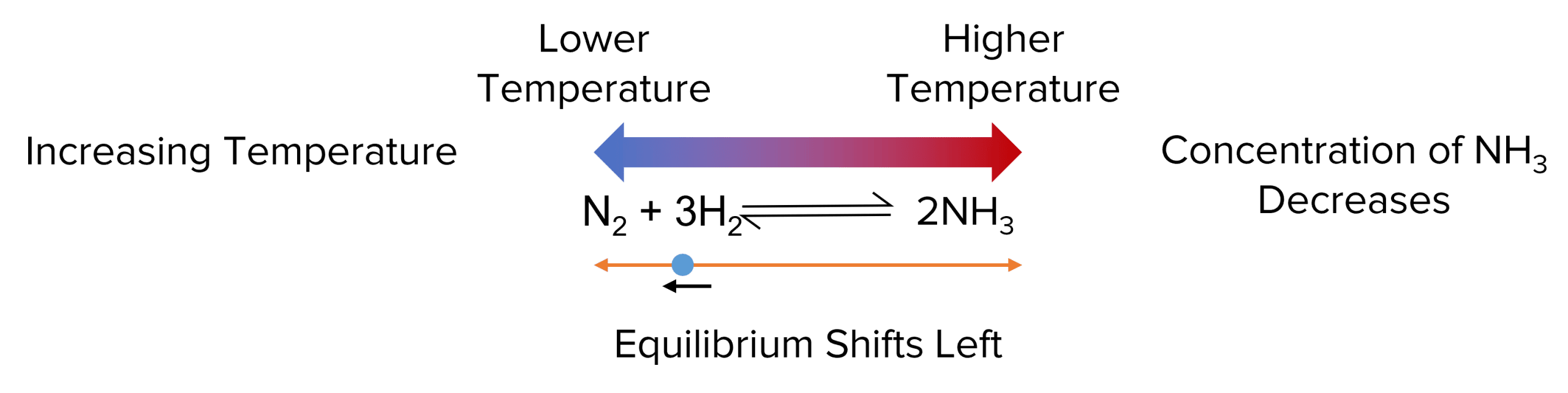
According to Le Chatelier’s principle, an equilibrium shift its position to oppose any change in its temperature. This means that, when the temperature is increased, the equilibrium will shift towards which ever side of the reaction has produces a lower temperature. This reduces the temperature of the reaction and opposes the change.
The enthalpy change of this reaction is negative, telling us that it is an exothermic reaction. This tells us that the right hand side of the equilibrium produces a higher temperature.
- If the temperature of the equilibrium is increased, the equilibrium will shift to oppose the change. To do this the position of the equilibrium shifts in the endothermic direction.
- In this case this shifts the equilibrium to the left hand side of the reaction. This shift will reduce the concentration of ammonia and increase the concentrations of hydrogen and nitrogen.
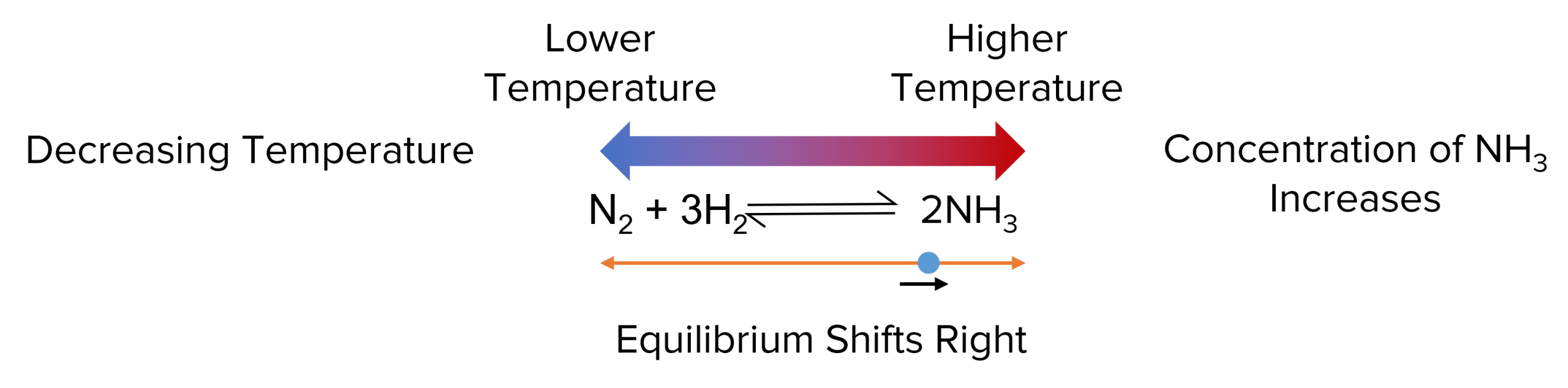
Conversely, if the temperature of the reaction is decreased the equilibrium will move to counteract this change by increasing the temperature. This leads the equilibrium to shift towards the exothermic side of the equilibrium. In this case, the equilibrium shifts right, increasing the concentration of \text{NH}_3.
Pressure
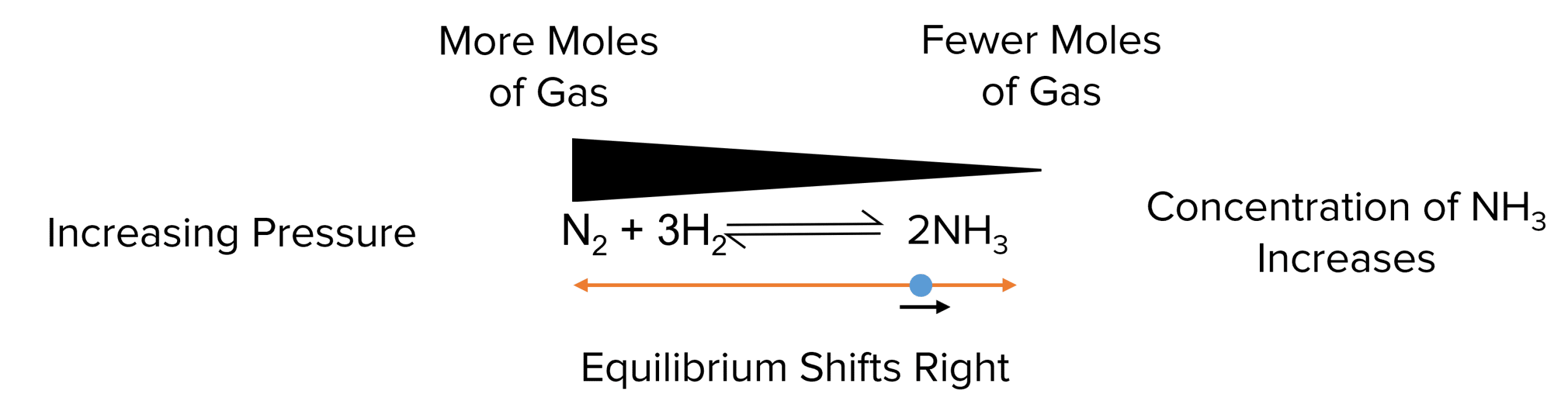
According to Le Chatelier’s principle, an equilibrium shift its position to oppose any change in its pressure. This means that, when the pressure is increased, the equilibrium will shift towards which ever side of the reaction has produces the fewest moles of gas. This reduces the pressure of the reaction and oppose the change.
Again we can look at the reaction of hydrogen and nitrogen to form ammonia. In this reaction, four moles of gas react on the left hand side to produce two moles of gas on the right. In this case, when the pressure of the equilibrium is increased, the equilibrium will shift to the right to decrease the number of moles in the system. This increases the concentration of \text{NH}_3 and decreases the concentration of \text{H}_2 and \text{N}_2.
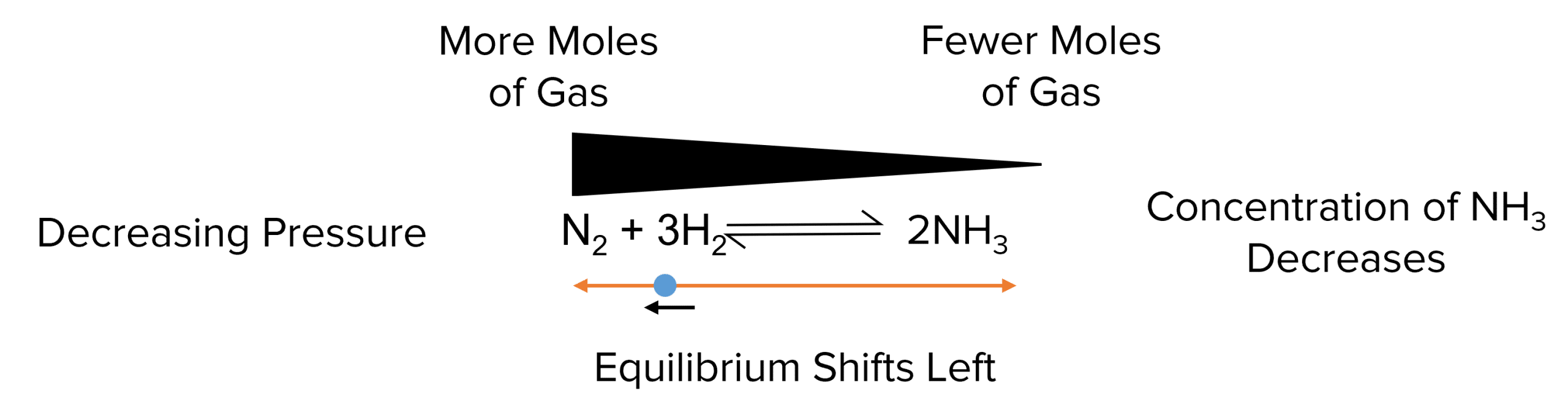
If the pressure is decreased, the equilibrium will shift left to increase the number of moles in the system. This decreases the concentration of \text{NH}_3.
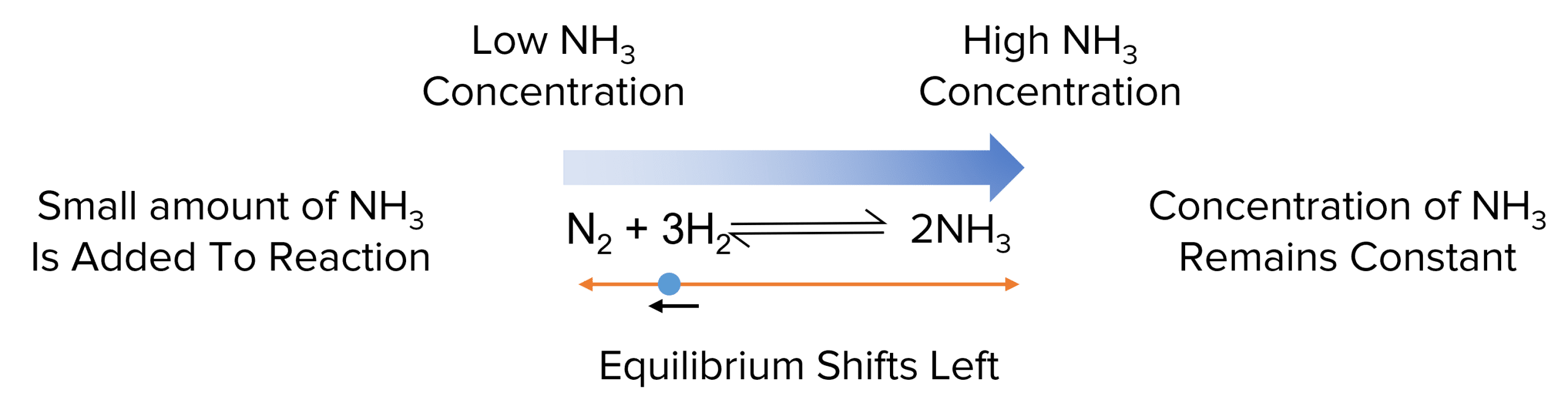
Concentration
In the case of the reaction above, if small volumes of ammonia are added to the equilibrium, increasing is concentration, it will shift towards the left to get rid of the excess.
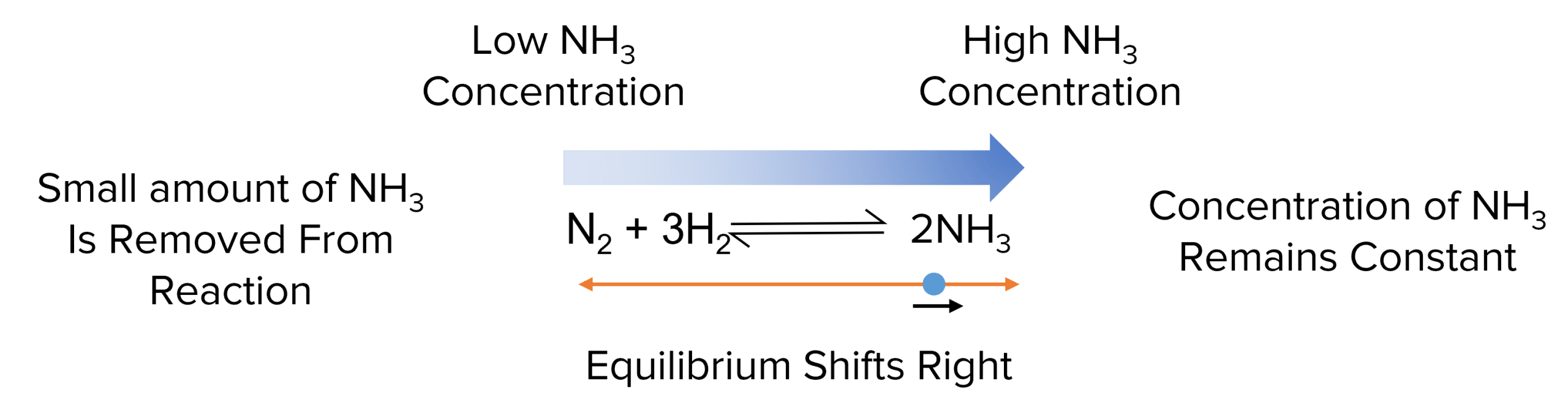
Similarly, if a small volume of ammonia is removed from the system, the equilibrium will shift to the right to make up for the loss.
Equilibria can only respond to small changes in the concentrations of reactants and products. If too much is added or removed then the equilibrium will breakdown before it can counteract the change.
Reversible Reactions and Equilibria Example Questions
Question 1: Define the term ‘Reversible Reaction’.
[2 marks]
A chemical reaction that can proceed in both the forward and backward direction.
Question 2: Define the term ‘equilibrium’.
[2 marks]
A system in which the forward and backwards reactions of a reversible reaction procced with equal rates.
Question 3: Predict and explain the effect on the position of the equilibrium of adding a small volume of hydrogen to the following equilibrium:
\text{H}_2+\text{I}_2\rightleftharpoons2\text{HI}
[3 marks]
- According to Le Chatelier’s Principle, the reaction will shift to oppose the addition of hydrogen.
- This means that the equilibrium will shift in the forward direction (or to the right) as this will react away the excess hydrogen.
Question 4: Predict the effect on the position of the equilibrium of increasing the pessure of the following equilibrium:
\text{H}_2+\text{I}_2\rightleftharpoons2\text{HI}
[3 marks]
- Changing the pressure of this reaction will have no effect.
- There is an equal number of gas moles on both sides of the equilibrium.
- As such neither side of the reaction will reduce the number of moles in the reaction so the pressure can’t be reduced.
Reversible Reactions and Equilibria Worksheet and Example Questions
Equilibrium
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now




