Chemical Reactions and Compounds
Chemical Reactions and Compounds Revision
Chemical Reactions and Compounds
Only stable elements, whose atoms have full outer electron shells, are found as single atoms. Most elements have atoms with incomplete outer electron shells, and these atoms form chemical bonds with other atoms in order to gain full outer shells and become stable. These chemical bonds can be broken or made in chemical reactions to produce different combinations of elements that we call compounds.
Chemical Reactions
In any chemical reaction, the starting chemicals are called reactants (also called reagents), and the chemicals made in the reaction are called products.
\text{Reactants} \rarr \text{Products}
During the chemical reaction, the chemical bonds in the reactants are broken and new chemical bonds are made to form the products. The atoms themselves remain unchanged in the chemical reaction, only the bonding changes.
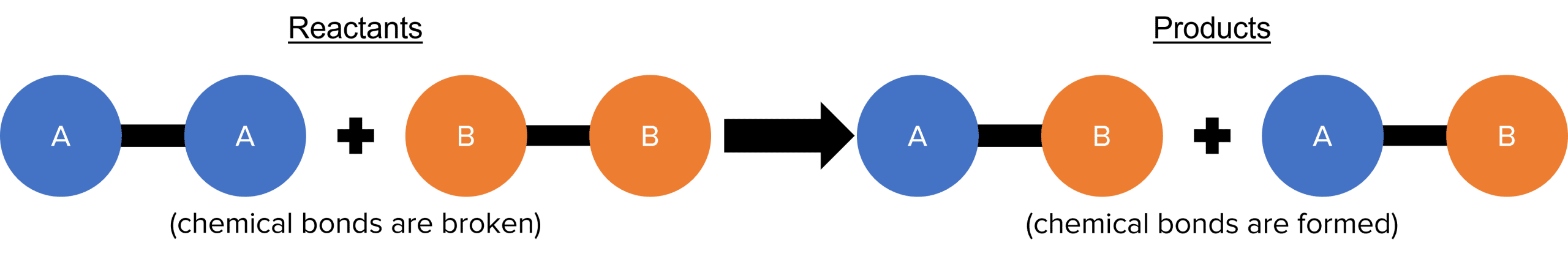
As the mass of the atoms comes primarily from their nuclei, and these are unchanged in the chemical reaction, the mass of the reactants is equal to the mass of the products. This is called conservation of mass. As the chemical reaction progresses, there will be observable and/or measurable changes, such as change in colour, temperature, flames, production of gas, production of solid precipitate, etc. Following the chemical reaction, the chemical and physical properties of the products will be different to those of the reactants.
Compounds
Substances that consist of one type of element chemically bonded together are called elemental compounds. One example would be “elemental” oxygen, which consists of two oxygen atoms chemically bonded together.

Substances that consist of two or more elements chemically bonded together are called compounds. One example would be carbon dioxide, which consists of two oxygen atoms chemically bonded to a carbon atom.
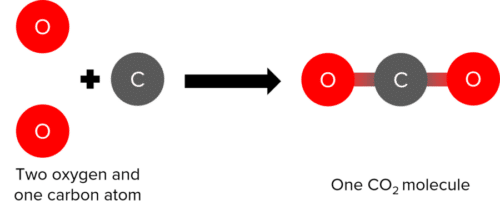
We represent elements and compounds with chemical formulae, with a specific, fixed ratio of each type of atom. For example, we would represent the two compounds above with the following formulae:
\text{(elemental) Oxygen: O}_2
\text{Carbon Dioxide: CO}_2
The numbers next to an element in a compound’s formula tell us how many of each atom there are in the ratio. For example, in the formula for carbon dioxide, there is a small 2 next to the symbol for oxygen and no number next to the symbol for carbon. This tell us that there are two oxygen atoms for every one carbon atom in the molecule. Changing the ratio of atoms in a chemical formula changes the chemical. It describes a substance with different chemical and physical properties. These numbers always found on the bottom right of the symbol.
\text{CO}_2 \text{ (carbon dioxide)} \rarr \text{CO (carbon monoxide)}
Chemical Equations
We can describe chemical reactions using various forms of chemical equation. Word equations involve writing the names of the reactants and compounds. Some chemicals have “common” names (like water for \text{H}_2\text{O}), whereas others are referred to with their chemical names (such as carbon dioxide for \text{CO}_2).
\text{Carbon} + \text{Oxygen} \rarr \text{Carbon Dioxide}
\text{Hydrogen} + \text{Oxygen} \rarr \text{Water}
Symbol equations show the chemical formulae of the reactants and products, thus showing the number of atoms of each element involved. Due to conservation of mass, symbol equations must be balanced so that the number of atoms of each element is the same before and after the chemical reaction. A useful technique to balance symbol equations is to use a tally table to record the number of atoms of each element involved.
\text{C} + \text{O}_2 \rarr \text{CO}_2
This symbol equation is balanced, as the number of carbon atoms and oxygen atoms is the same on each side of the equation:
| Left Hand Side | Right Hand Side | ||
| C | O | C | O |
| 1 | 2 | 1 | 2 |
\text{H}_2 + \text{O}_2 \rarr \text{H}_2\text{O}
This symbol equation is not yet balanced, as there are 2 oxygen atoms in the reactants, and only 1 in the products.
| Left Hand Side | Right Hand Side | ||
| H | O | H | O |
| 2 | 2 | 2 | 1 |
We need to balance the equation, but we cannot change the ratio of atoms in the chemicals. Instead, we can change how many of each chemical we have, by adding multiples of chemicals as necessary. If we place the number 2 in front of \text{H}_2\text{O}, we multiply all of the atoms in that chemical by 2, which changes the tally:
\text{H}_2 + \text{O}_2 \rarr \textcolor{#008d65}{2} \text{H}_2\text{O}
| Left Hand Side | Right Hand Side | ||
| H | O | H | O |
| 2 | 2 | 4 | 2 |
Oxygen is now balanced, but hydrogen is not. There are 4 hydrogen atoms in the products, and 2 in the reactants, so we need to multiply the hydrogen in the reactants by 2 as well:
\textcolor{#008d65}{2}\text{H}_2 + \text{O}_2 \rarr 2\text{H}_2\text{O}
| Left Hand Side | Right Hand Side | ||
| H | O | H | O |
| 4 | 2 | 4 | 2 |
The equations is now balanced.
Chemical Reactions and Compounds Example Questions
Question 1: What is meant by “conservation of mass”?
[1 mark]
The mass of the reactants is always equal to the mass of the products (in a chemical reaction).
Question 2: How do the products of a chemical reaction differ from the reactants, in terms of properties and bonding?
[2 marks]
New chemical bonds between atoms, and different physical and chemical properties.
Question 3: Balance the symbol equation for this chemical reaction.
\text{CH}_4 + \text{O}_2 \rarr \text{CO}_2 + \text{H}_2\text{O}
\text{CH}_4 + \text{O}_2 \rarr \text{CO}_2 + \text{H}_2\text{O}
| Right Hand Side | Left Hand Side | ||||
| C | H | O | C | H | O |
| 1 | 4 | 2 | 1 | 2 | 3 |
\text{CH}_4 + \underline{\textcolor{#008d65}{2}}\text{O}_2 \rarr \text{CO}_2 + \underline{\textcolor{#008d65}{2}}\text{H}_2\text{O}
| Right Hand Side | Left Hand Side | ||||
| C | H | O | C | H | O |
| 1 | 4 | 4 | 1 | 4 | 4 |

MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



