Elements
Elements Revision
Elements
We have seen how the nucleus of an atom contains protons and neutrons, and that the nuclei of different atoms have different numbers of protons and neutrons. The differences in proton number (also called atomic number) result in the different elements, and the differences in neutron number result in the different isotopes of elements.
The Chemical Elements
There are roughly 100 different types of atom, called the elements. The proton number of an atom determines which element it is. If two atoms have the same proton number, they are atoms of the same element. If two atoms have different proton numbers, they are atoms of different elements. We represent elements with a chemical symbol, either a capital letter, or a capital and lowercase letter.

Some chemical symbols are derived from the Latin or Greek names of elements, which is why certain elements have chemical symbols that seem unrelated to their English names
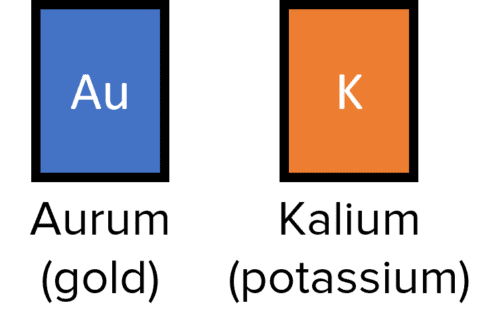
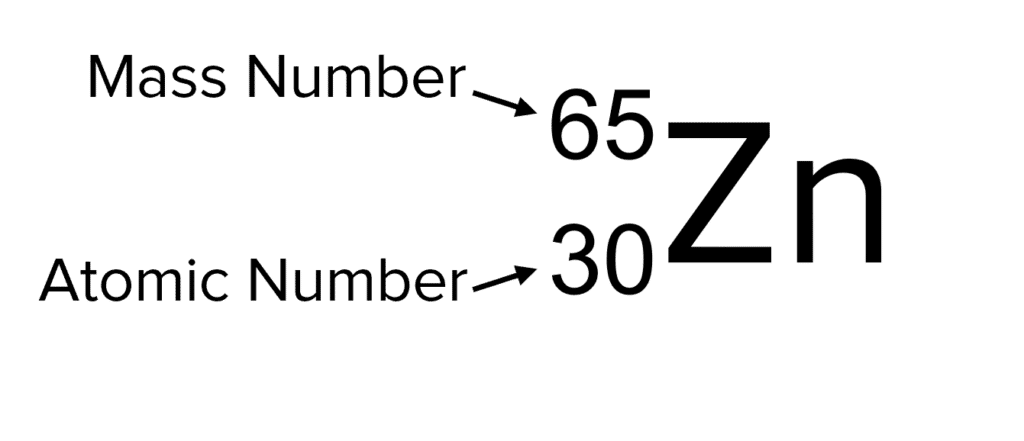
The elemental chemical symbol typically accompanies the mass number and proton number when elements are displayed. Usually the mass number is displayed above the proton number, but if in doubt remember that the mass number (unless there are no neutrons in the nucleus) will be larger than the proton number.
Isotopes
Atoms of the same element (having the same proton number) can have different numbers of neutrons – we call these isotopes. This means that the atomic masses of isotopes differ, which also causes their physical properties to be slightly different. As elements have the same number of electrons as isotopes of the same the element, the chemical properties of the isotopes of an element are the same as those of the element. Elements can have multiple isotopes, but the abundance of each isotope (the proportion of all the atoms of that element that exist as each isotope) varies.
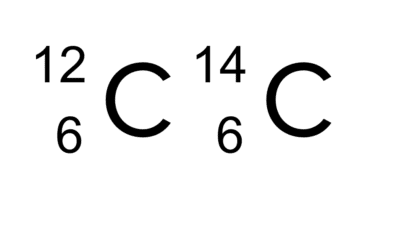
Relative Atomic Mass
In order to make certain chemical calculations, we need to know the atomic mass of the elements involved, but isotopes complicate this. The solution is to use relative atomic mass – an averaged value of atomic mass which incorporates both the abundance and mass number of all known isotopes. The relative atomic mass of an element is referred to as the element’s Ar, and can be calculated as follows:
\text{Relative Atomic Mass}=\frac{(\%\text{ Isotope }1\times\text{Mass Isotope }1 )+(\%\text{ Isotope }2\times\text{Mass of Isotope }2)+(\%\text{ Isotope }3\times\text{Mass of Isotope }3)\cdots}{\text{Sum of All Isotope Abundances}}
When abundances are given in percentages, the sum of the abundances will always be 100\%.
For example, find the relative atomic mass for element X, using the following data:
| Element X | Isotope 1 | Isotope 2 | Isotope 3 | Isotope 4 |
| Mass Number | 10 | 8 | 11 | 13 |
| Abundance (\%) | 50 | 20 | 20 | 10 |
\text{Relative Atomic Mass} = \frac{(10\times50)+(8\times20)+(11\times20)+(13\times10)}{100}=\textcolor{#008d65}{10.1}
Because relative atomic masses are averages, they are often not whole numbers. Instead, most if not all elements have relative atomic masses that include decimal numbers.
Elements Example Questions
Question 1: Identify which of the following symbols represent isotopes of the same element:
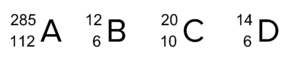
[1 mark]
B and D as the proton number is the same.
Question 2: Which of the following is not an isotope of chlorine?
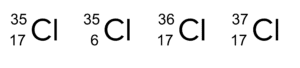
[1 mark]

The mass number is the same but the atomic number is not.
Question 3: Calculate the relative atomic mass of element Z using the following data:
| Element Z | Isotope 1 | Isotope 2 | Isotope 3 |
| Mass Number | 30 | 32 | 36 |
| Abundance (\%) | 45 | 20 | 35 |
[4 marks]
\text{Relative Atomic Mass}=\frac{(30\times45)+(32\times20)+(36\times35)}{100}
=\frac{1350+640+1260}{100}
=\frac{3250}{100}=32.5

MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



