The History of Atomic Structure
The History of Atomic Structure Revision
The History of Atomic Structure
Scientific theories and models, based on experimental data, are used to understand the natural world. If new evidence contradicts established theories and models, they are changed or replaced. Our understanding of atomic structure is a classic example of this.
Changing Models
The accepted model of atomic structure has evolved over time, and the flowchart below represents the most important stages. Each model was for a time accepted as correct. However, each model was eventually replaced by a newer one thanks to the discovery of new evidence. This led to increasingly complex models of atomic structure.
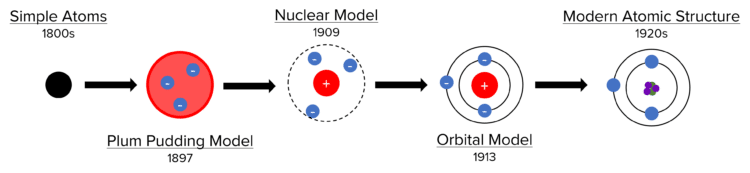
Simple Atoms
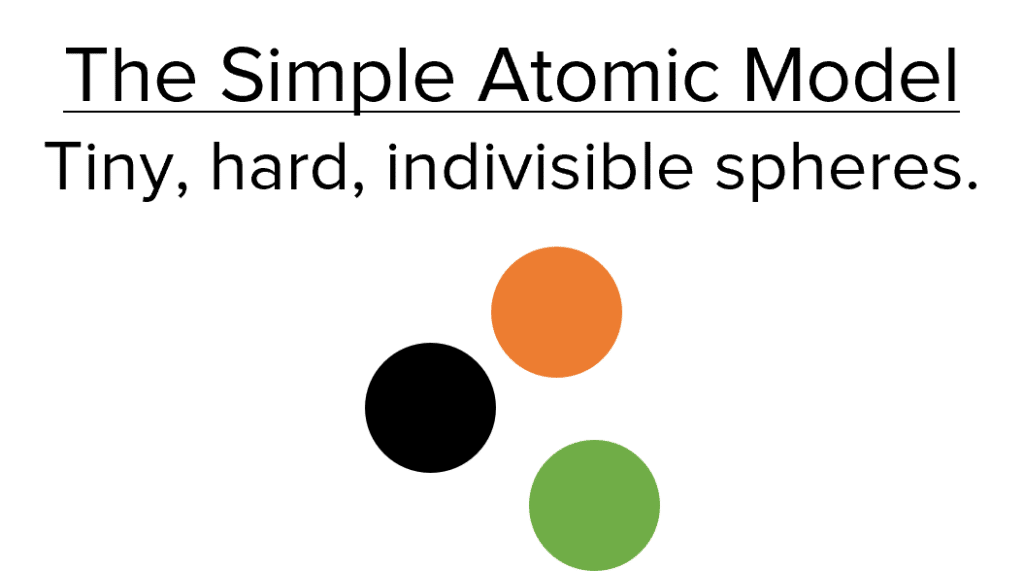
The earliest concepts of atomic theory considered all matter to be made of small, indivisible, spherical atoms. These ideas began in Ancient Greece, but were fully adopted into Chemistry in the 1800’s. Due to a lack of contradictory evidence, these ideas persisted for a long time, until JJ Thomson discovered the first known subatomic particle – the electron. Atoms could no longer be considered as indivisible spheres, so the accepted model changed to the Plum Pudding model.
The “Plum Pudding”
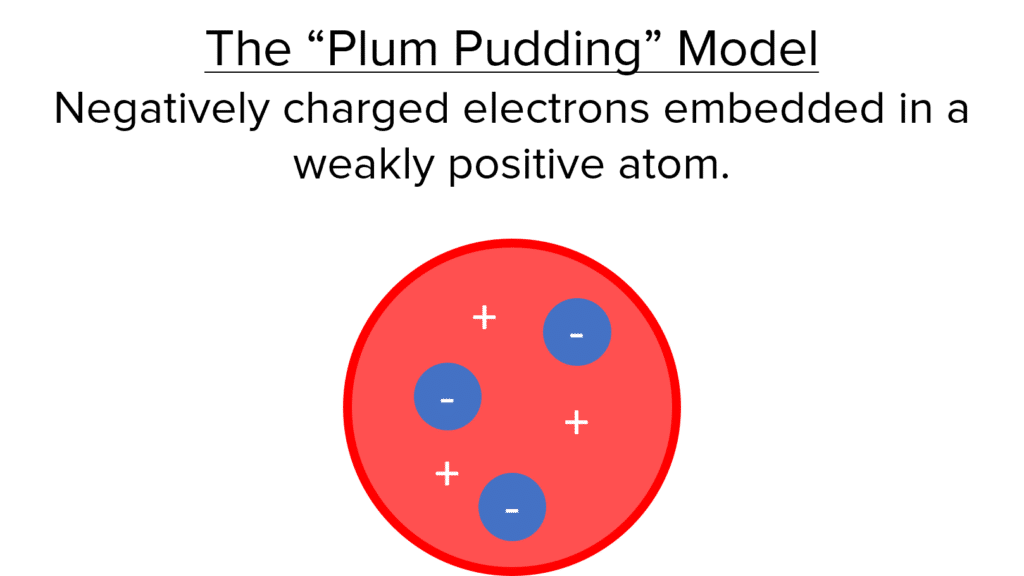
In this model, the atom is considered to be a solid sphere with a weakly positive charge. Negatively charged electrons are embedded into this solid sphere (like plums in a plum pudding – hence the name). The Plum Pudding model persisted until Ernest Rutherford discovered the existence of the nucleus in the alpha particle scattering experiment (detailed below). With the discovery of the nucleus, atoms could no longer be considered as solid spheres, so the accepted model changed to the Nuclear model.
The Nuclear Model
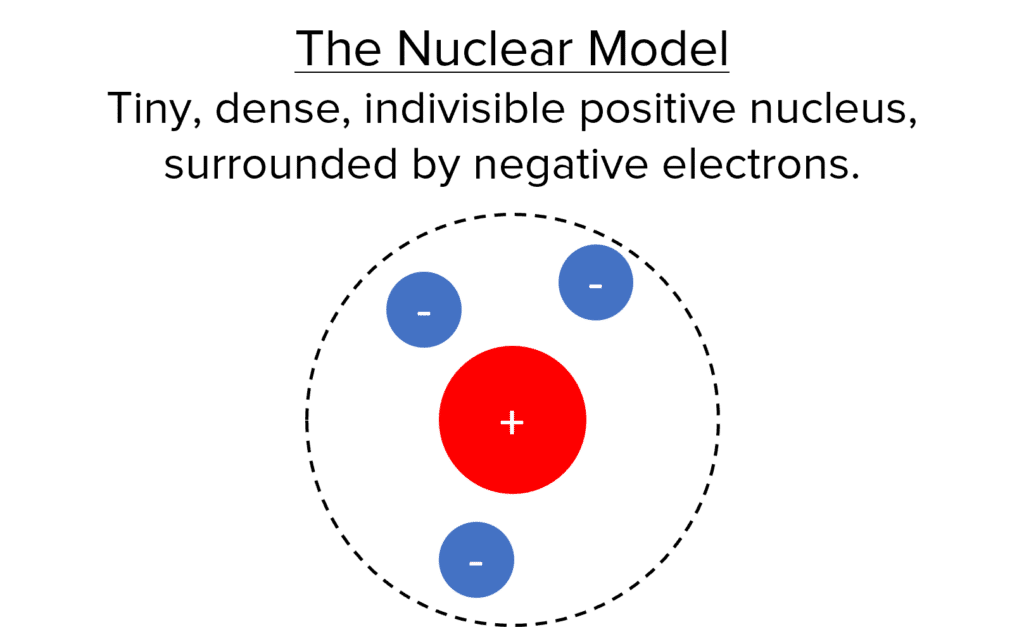
In the Nuclear model, atoms consist of a tiny central nucleus that is dense, indivisible and strongly positively charged. The nucleus is surrounded by negatively charged electrons. Most of the atom in this model is actually empty space, with the nucleus containing effectively all of the mass of the atom. Niels Bohr’s calculations suggested that the Nuclear model would be unstable if electrons simply surrounded the nucleus. His predictions were verified by experimental data, so the accepted model changed to the Orbital model.
Orbitals
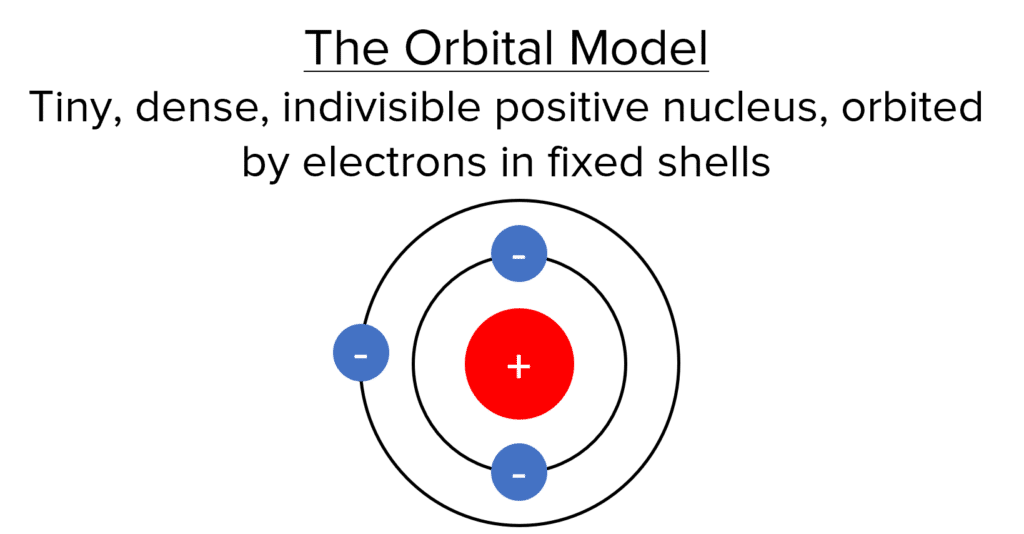
In the Orbital model, the tiny, dense, indivisible, positive nucleus remains, but the surrounding electrons orbit in electron shells that are at fixed distances from the nucleus. The energy of electrons is lower in the shells closer to the nucleus.
Modern Atomic Structure
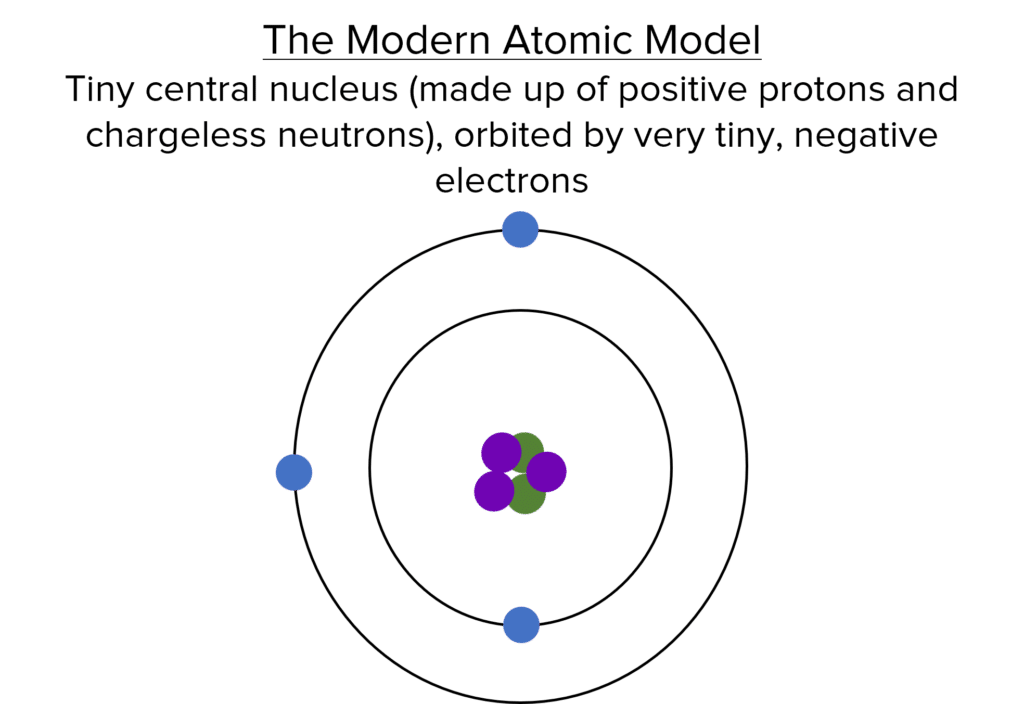
Rutherford subsequently discovered that the positive charge of the nucleus could be divided into equally charged units, which he called protons.
Chadwick then discovered that the additional mass of the nucleus could be divided into equal units with no electrical charge, which he called neutrons.
These two discoveries showed that the nucleus itself was not indivisible, so the accepted model had to change. The subsequent model is the atomic structure that we have previously seen – a central nucleus consisting of protons and neutrons, surrounded by electrons in electron shells.
As we have seen, this model has developed over time, through the repeated revision of earlier models based on new experimental evidence. This gives us an insight into how scientific knowledge progresses over time.
The Alpha Particle Scattering Experiment
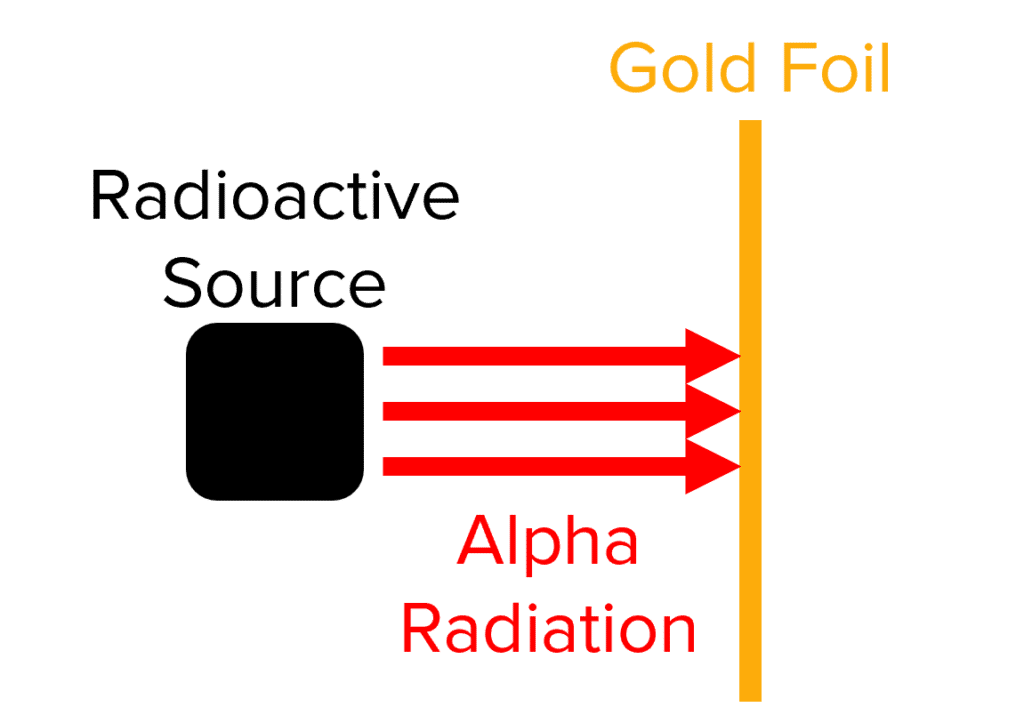
This classic experiment provided the evidence that led to the replacement of the Plum Pudding model with the Nuclear model. The experiment involved a radioactive material, and a thin sheet of gold foil.
The alpha radiation – made up of small particles called alpha (\alpha) particles – emitted by the radioactive material is positively charged and has a (relatively) large mass. If the Plum Pudding model was correct, the alpha radiation should easily pass straight through the gold foil and be detected on the other side. This is because the weakly positive, solid atoms in the Plum Pudding model have neither sufficient density to resist the radiation, nor sufficiently strong positive charge to deflect the radiation.

However, Rutherford obtained very unexpected results. Most of the radiation did pass through the gold foil as expected, but some was significantly deflected, and some actually reflected back towards the radioactive material.
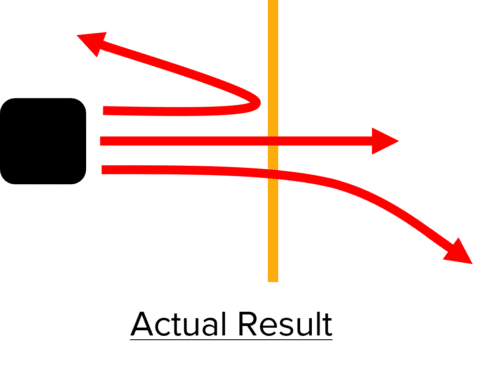
The Plum Pudding model could not explain these results, so Rutherford designed the nuclear model to interpret his experimental data. The nuclear model allowed for three different scenarios which could explain the alpha scattering observed.
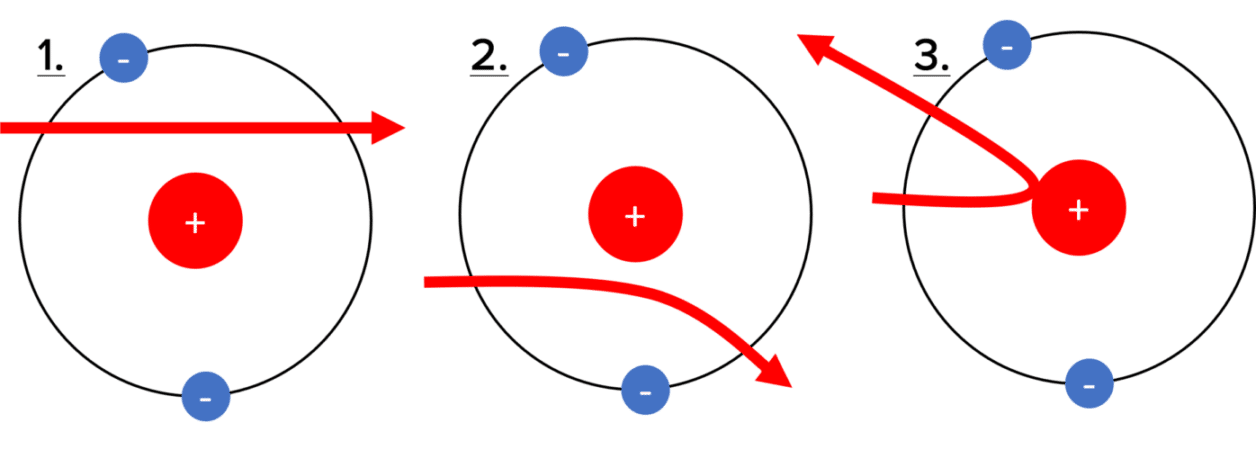
1. The alpha radiation passes straight through the foil. The radiation is able to pass through the atom without colliding with anything or being deflected. This is the most common scenario, indicating that there is lots of empty space within the foil for the radiation to pass through. Therefore the atom must be made up mostly of empty space.
2. The alpha radiation is deflected through the foil. This implies that the positively charged alpha radiation is being repelled by another positive charge. As we already know that the atom is mostly empty space, we can deduce that the alpha radiation is being deflected by a tiny but strongly positively charged particle at the centre of the atom. This tells us the atom must have a tiny central nucleus that has a strong positive charge.
3. The alpha radiation is reflected back towards the source. We can deduce that the relatively heavy alpha radiation is bouncing back off the nucleus in the centre of the atom. This tell us that the nucleus must be very dense.
The History of Atomic Structure Example Questions
Question 1: Describe the features of the atom as imagined in the “plum pudding” model.
[3 marks]
Electrons with a strong negative charge that are embedded inside a weakly positive and solid nucleus.
Question 2: Describe one similarity and two differences between the Plum Pudding model and the Nuclear model of atomic structure
[3 marks]
Similarities
- both contain negatively charged electrons / Both contain positive charge.
Differences
- PPM has weakly positive atom, whereas NM has a tiny strongly positive nucleus.
- PPM has embedded electrons, whereas NM has electrons orbiting the nucleus.
- Plum pudding model has a solid atom, whereas nuclear model has most of the atom being empty space with the mass in the nucleus.
Question 3: Explain how the evidence from the “Alpha Particle Scattering” experiment led to the Nuclear model.
[6 marks]
The experiment saw:
- Some radiation passed through the gold foil which showed that most of the atom is empty space.
- Some radiation deflected as it passed through the gold foil which showed that the nucleus is strongly positively charged.
- Some radiation reflected back from the gold foil which shows that the nucleus is dense and contains the mass of the atom.