Atoms
Atoms Revision
Atoms
All matter is made of atoms. However, atoms themselves are made of smaller subatomic particles called protons, neutrons and electrons. Each of these subatomic particles have certain properties, and the specific number and arrangement of each subatomic particle gives an atom its mass number, atomic number and electronic structure.
Atomic Structure
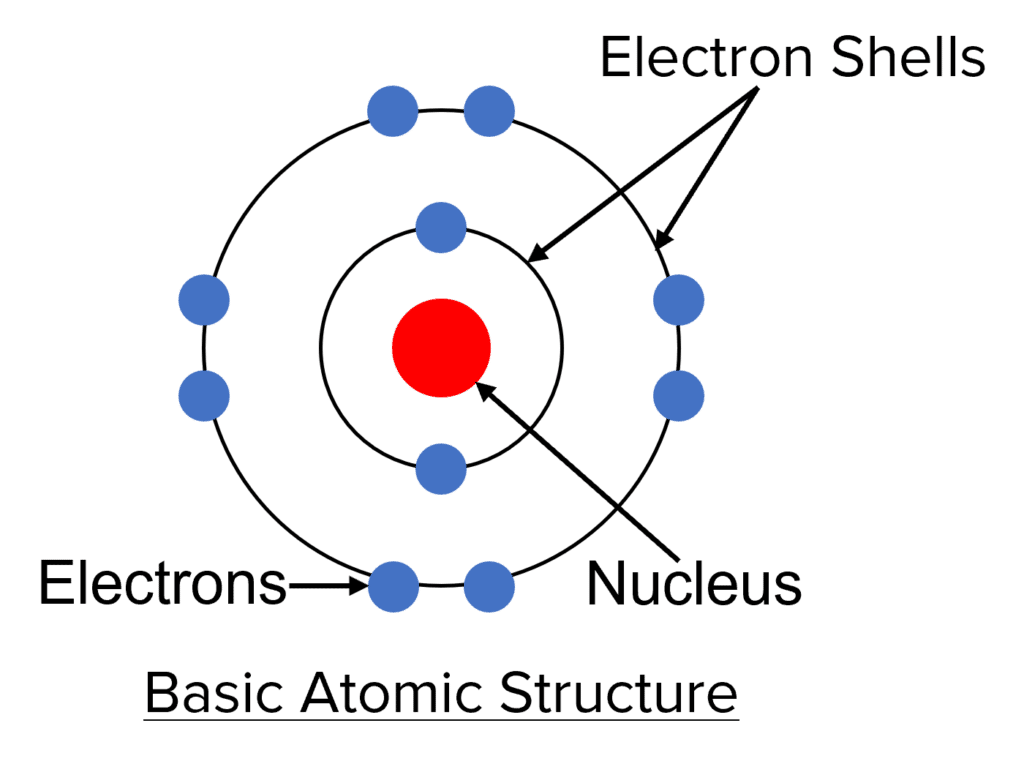
- Atoms are very small – approximately 0.1 nanometers in diameter \left(1 \times 10^{-10}\text{ metres}\right). In a typical grain of sand there are around 10^{19} atoms!
- The basic structure of any atom consists of a tiny, dense, central nucleus (approximately 1\text{/}10000 the size of the atom) surrounded by electron shells (sometimes called energy levels).
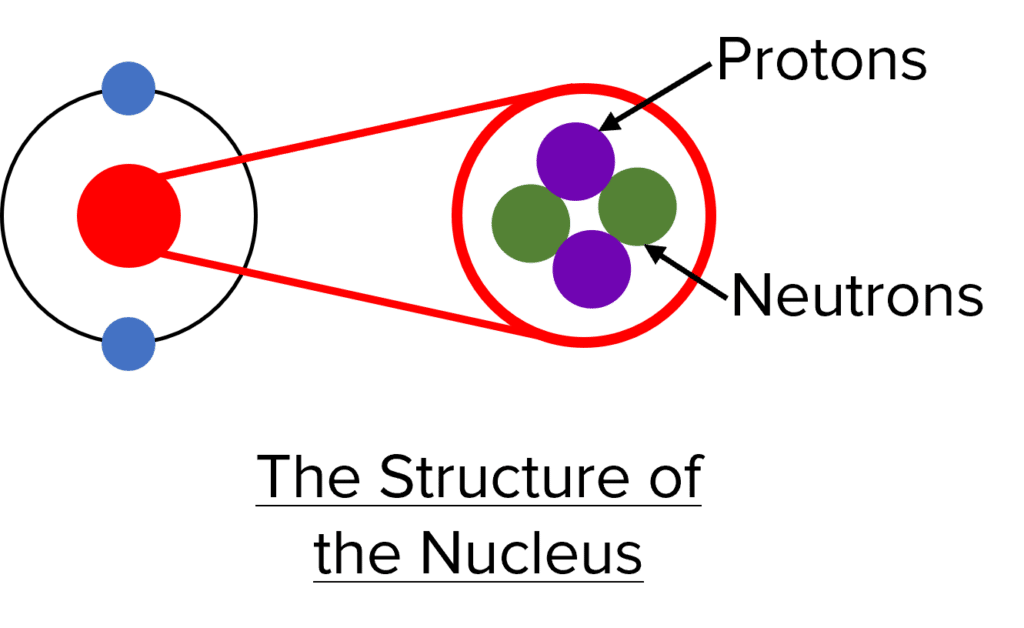
- The nucleus contains an atom’s protons and neutrons, while an atom’s electrons orbit the nucleus in the electron shells.
These three subatomic particles have a different relative charge and relative mass, summarised in the table below:
| Sub-Atomic Particle | Relative Charge | Relative Mass |
| Proton | \textcolor{#7105b3}{+1} | \textcolor{#7105b3}{1} |
|
Neutron |
\textcolor{#548235}{0} | \textcolor{#548235}{1} |
|
Electron |
\textcolor{#4472c4}{-1} | \textcolor{#4472c4}{\text{(essentially) }0} |
Different atoms have different numbers of protons, neutrons and electrons. In any individual atom, the number of protons is equal to the number of electrons, so atoms have no overall electrical charge (positive charges are cancelled out by an equal number of negative charges).
Atomic Number and Mass Number
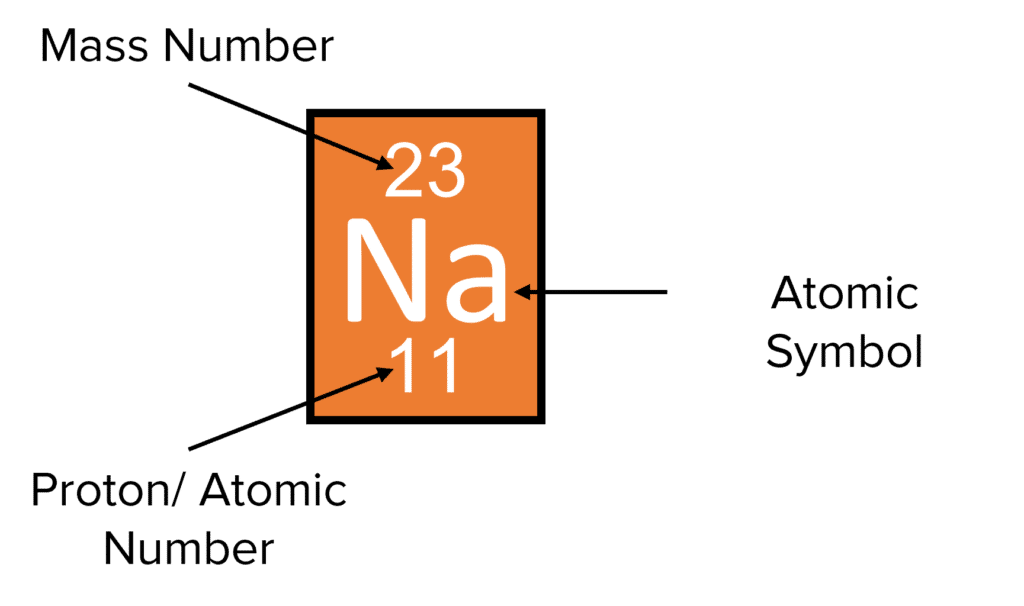
As the table above shows, effectively all the mass of the atom comes from the protons and neutrons, so the sum of the number of protons and the number of neutrons gives the mass number of an atom. The number of protons in an atom is called the proton number or atomic number. We represent the proton number and mass number of an atom numerically. For example, Sodium (Na) has 11 protons and 12 neutrons:
Because the proton number is 11, we also know that sodium will have 11 electrons, as the proton number equals the electron number. Note that (unless there are no neutrons in the nucleus) the mass number is always larger than the proton number, as it combines the number of protons and the number of neutrons. We can therefore calculate the number of protons, neutrons and electrons in any atom if we know the proton number and mass number.
Electronic Structure
Electrons orbit the nucleus in electron shells, which have fixed distances from the nucleus. Electron shells that are closer to the nucleus house lower energy electrons. Different atoms have different numbers of electrons, and so have different numbers of electron shells. The arrangement of an atom’s electrons in its electron shells can be understood using the following rules (which apply for the 20 simplest atoms):
-
-
- Each electron shell must be filled before a new shell can begin to be filled up.
- The first electron shell can hold a maximum of 2 electrons.
- Every subsequent electron shell can hold a maximum of 8 electrons.
-
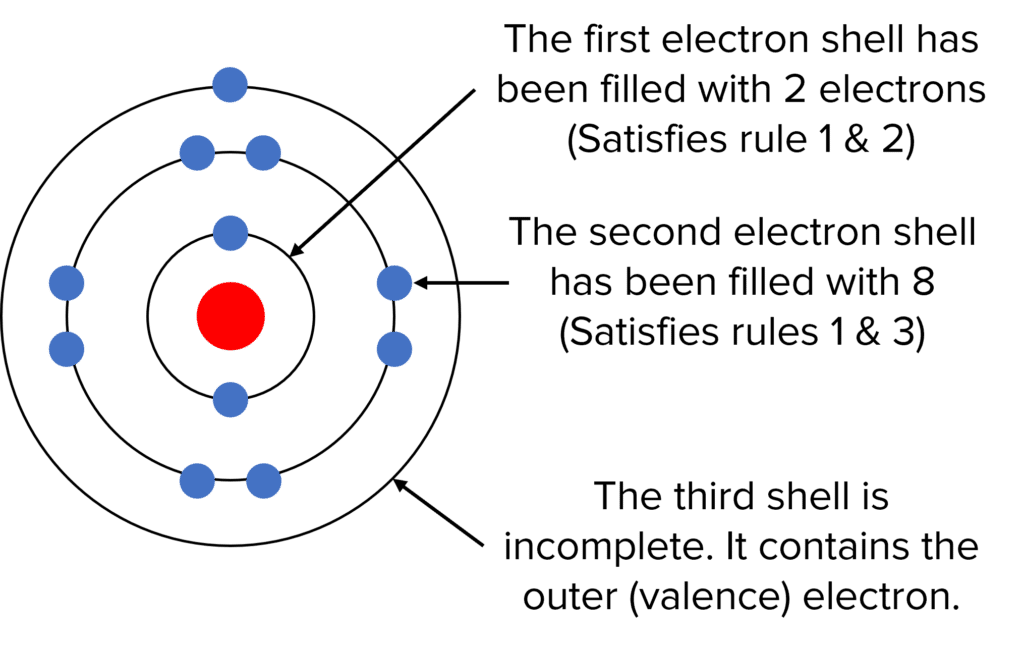
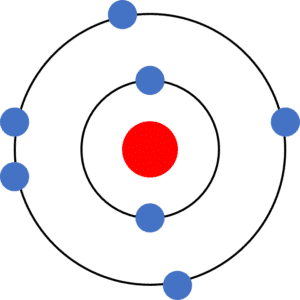
Following these rules, we can draw the electronic structure (sometimes called the electronic configuration) of an atom which has 11 electrons, as shown to the right:
We could also write the electronic structure of the atom as 2\text{,} 8\text{,} 1. Each number, separated by a comma, tells us how many electrons are in each electron shell; 2 in the first electron shell, 8 in the second electron shell, and 1 in the third (outer) electron shell.
Atoms with incomplete outer electron shells are unstable. They will react in chemical reactions with other atoms to gain a complete outer electron shell and so become stable. This is the basis of all of Chemistry.
Atoms Example Questions
Question 1: What two particles make up the tiny central nucleus of an atom?
[2 marks]
Protons and neutrons
Question 2: Atom “W” has 12 protons and 14 neutrons. Give the mass number of atom W.
[2 marks]
\text{Mass Number} = 12 + 14 = \underline{26}
Question 3: Atom “V” has seven electrons and an electronic structure of 2,5. Draw the correct electronic structure for atom “V”
[2 marks]