Endothermic and Exothermic Reactions
Endothermic and Exothermic Reactions Revision
Endothermic and Exothermic reactions
When chemical reactions happen, there is a change in energy. These changes in energy can either cause a reaction to use up or to produce heat. These changes in energy can be harnessed for a range of uses and can be studied using graphs called reaction profiles. By looking at the reaction profile of a given chemical process we can determine whether it is exothermic or endothermic.
Energy Changes
Chemical reactions are almost always accompanied by changes in energy. All chemicals store energy, and different chemicals will store different amounts. As such, when chemicals change occur, the amount of energy in the system will change. This energy change must be accompanied by a either an absorption or a release of energy from the system. This is a result of a principle called the conservation of energy. The principle states that:
Energy is neither created nor destroyed. It can only be transferred from one form to another.
In practice this means that if a chemical reaction causes the system to lose energy then this energy must have been transferred to the surroundings. When talk about energy changes, the system and the surroundings have a defined meaning. In this case, the system is the chemical reaction being studied. The surroundings can be taken as the reaction vessel in which the chemical reaction takes place. The universe is a combination of the system and the surroundings.
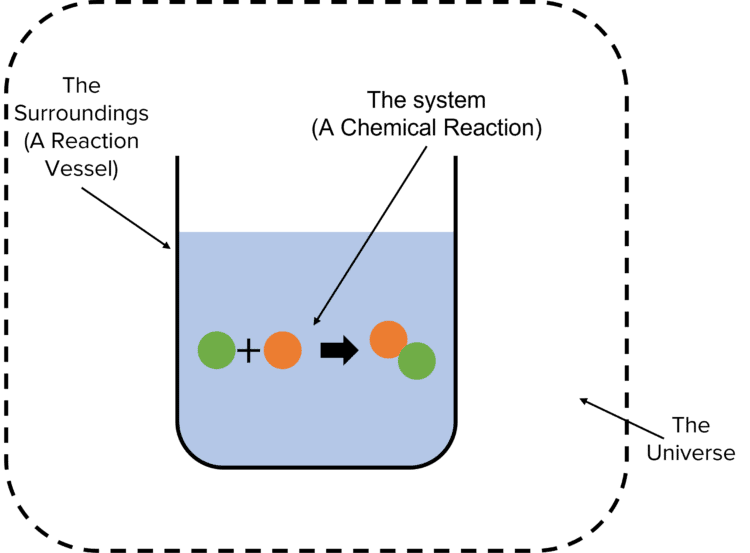
We can state the principle of the conservation of energy in terms of the universe:
The total energy of the universe is constant.
According to the principle of conservation, when a chemical reaction transfers energy to its surroundings the energy must come from the chemicals involved. This means that the energy of the chemical reactants must decrease by an amount equal to the energy transferred. The changes in the energy of the chemicals involved is accounted for by the difference in the energy stored by the reactants and the products. We can define two types of reaction depending on whether the reactants or the products have the highest energy.
Endothermic and Exothermic Reactions
Chemical reactions may be classified according to the energy changes that they produce. Chemical reactions will either release or absorb energy to and from their surroundings. This allows us to define reactions as either exothermic or endothermic.
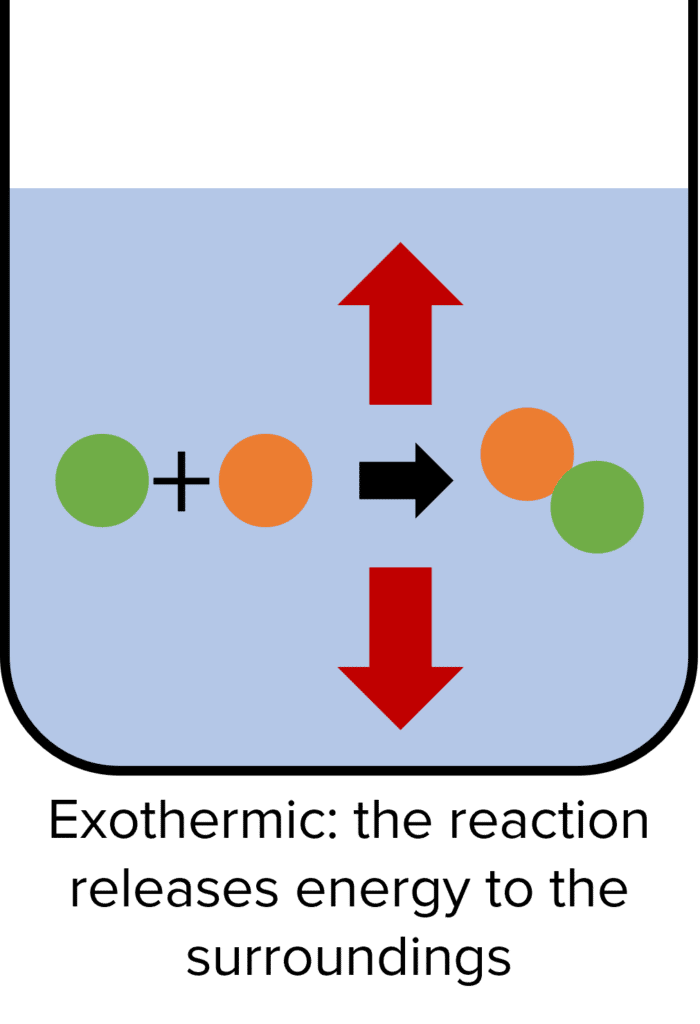
Exothermic Reactions
Exothermic reactions are chemical reactions that release energy to their surroundings. Reactions are exothermic when the energy stored by the reactants is more than the energy stored by the products. As such there is a release of energy as we move from reactants to products because, according to the conservation of energy, this energy cannot be destroyed. The energy released will be equal to the difference between the energy of the reactants and the energy of the products.
When exothermic reactions take place, the temperature of the surroundings increases as a result of the release of energy. Examples of exothermic reactions are combustion, neutralisation of an acids and bases, and the freezing of water.
Handwarmers and self heating camping meals make use of exothermic chemical reactions to produce a temperature change without requiring any external energy.
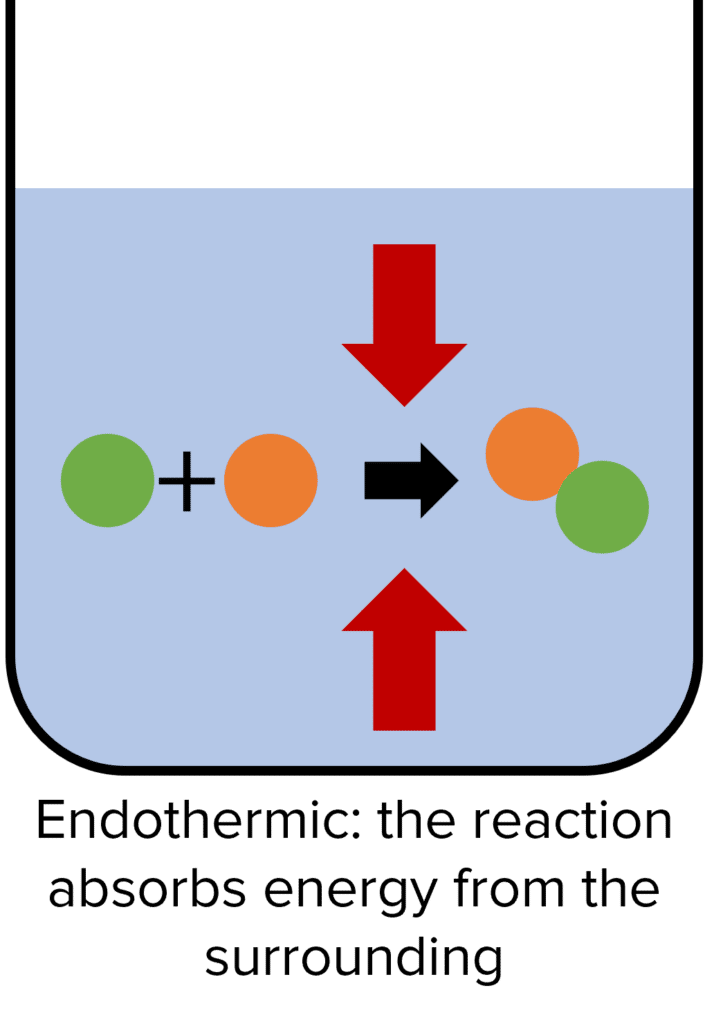
Endothermic Reactions
Endothermic reactions are chemical reactions that absorb energy from their surroundings. In these reactions, the energy stored by the reactants is less than the energy stored by the products. This means that the energy has to be absorbed for the reaction to happen. Because energy cannot be created it must be transferred into the chemical reaction by the surroundings. The energy absorbed by the chemical reaction will be equal to the difference between the energy of the products and the energy of the reactants.
As energy is being absorbed from the surroundings in endothermic reactions, the temperature of the surroundings decreases during the reaction. Examples of endothermic reactions include thermal decomposition reactions.
One practical use of endothermic reactions is in sports injury packs.
The type of a chemical reaction can be identified by measuring the temperature of the reaction vessel over time. If the temperature increases then the reaction is exothermic. If the temperature decreases then the reaction is endothermic. It is also possible to tell what type of reaction is happening by looking at its reaction profile.
Reaction Profiles
Reaction profiles (also known as energy diagrams) show us how the energy of the system changes during a chemical reaction. By looking at these profiles we can tell whether a reaction is endothermic or exothermic. We can also see how much energy is needed for a reaction to take place at all. This energy is known as the activation energy and is the energy with which particles need to collide to be able to react.
Exothermic Reaction Profiles
Reactions that are exothermic are characterised by systems where there is more energy stored in the reactants than in the products. This means that, in the energy profile, the level at which we draw the energy of the reactants is higher along the y-axis than that of the products. The difference in height between them will be equal to the energy released by the reaction to its surroundings.
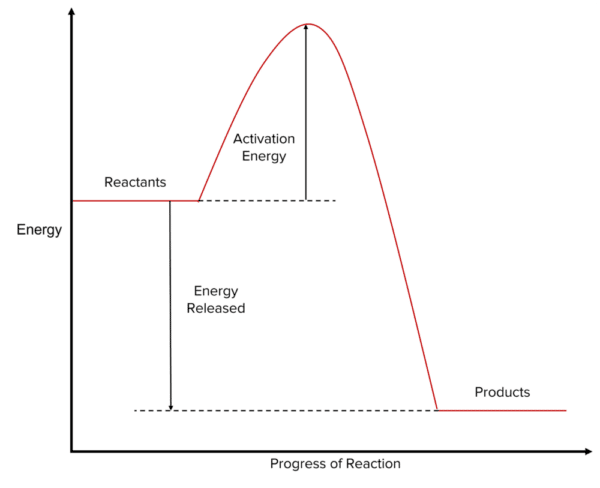
The reactants and products are separated by a hump in the reaction profile. The difference in height between the top of the hump and of the energy of the reactants is equal to the activation energy of the reaction. For a reaction to take place the reactants must gain enough energy to overcome this hump.
In exothermic reactions the overall change in the energy of the reaction is negative as there is a decrease in energy between the products and the reactants.
Endothermic Reaction Profiles
Endothermic reactions are characterised by systems in which more energy is stored in the products than in the reactants. In the energy profile of an endothermic reaction, the level at which we draw the energy of the reactants is lower along the y-axis than that of the products. The difference in height between the reactants and products is equal to the energy absorbed by the reaction from the surroundings. In these reactions the overall energy change of the system is positive as their is an increase in energy between the reactants and products.
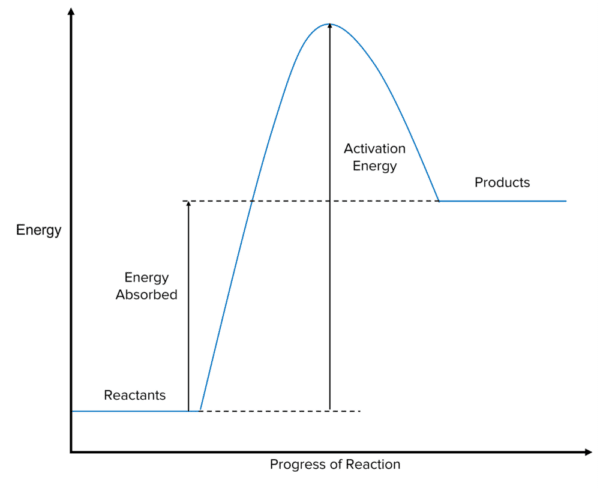
Bond Energies
So far on this page energy changes have been discussed in terms of the energy stored by the chemicals in a reaction. This energy is typically considered as what are known as bond energies. Bond energies are the energy stored within a chemical bond. When a chemical bond is made energy is released to the surroundings. When a chemical bond is broken energy must be absorbed from the surroundings.
Every chemical bond will have a specific chemical energy associated with it (varying slightly depending upon the compound in which it is found) and these bond energies can be used to calculate the overall energy change of a reaction. To do this, we subtract the energy of the bonds formed by that of the bonds broken.
\text{Energy Change}=\text{Sum of Bonds Broken}-\text{Sum of Bonds Made}
If the energy change for a given reaction is negative, more energy is released by the bonds formed in the products than is used in breaking the bonds of the reactants. This means that the reaction releases energy over all. As such, if the energy change is negative, the reaction is exothermic. When the energy change is positive, the energy released in making bonds is less than what is used to break bonds and so the reaction absorbs energy. This means it that the reaction is endothermic.
Required Practical
Measuring the temperature change of a reaction
By mixing reactants in an insulated cup it is possible to determine the effects of changing conditions on the temperature change of a reaction. In this experiment, the effects of concentration of acid on temperature changes are measured.
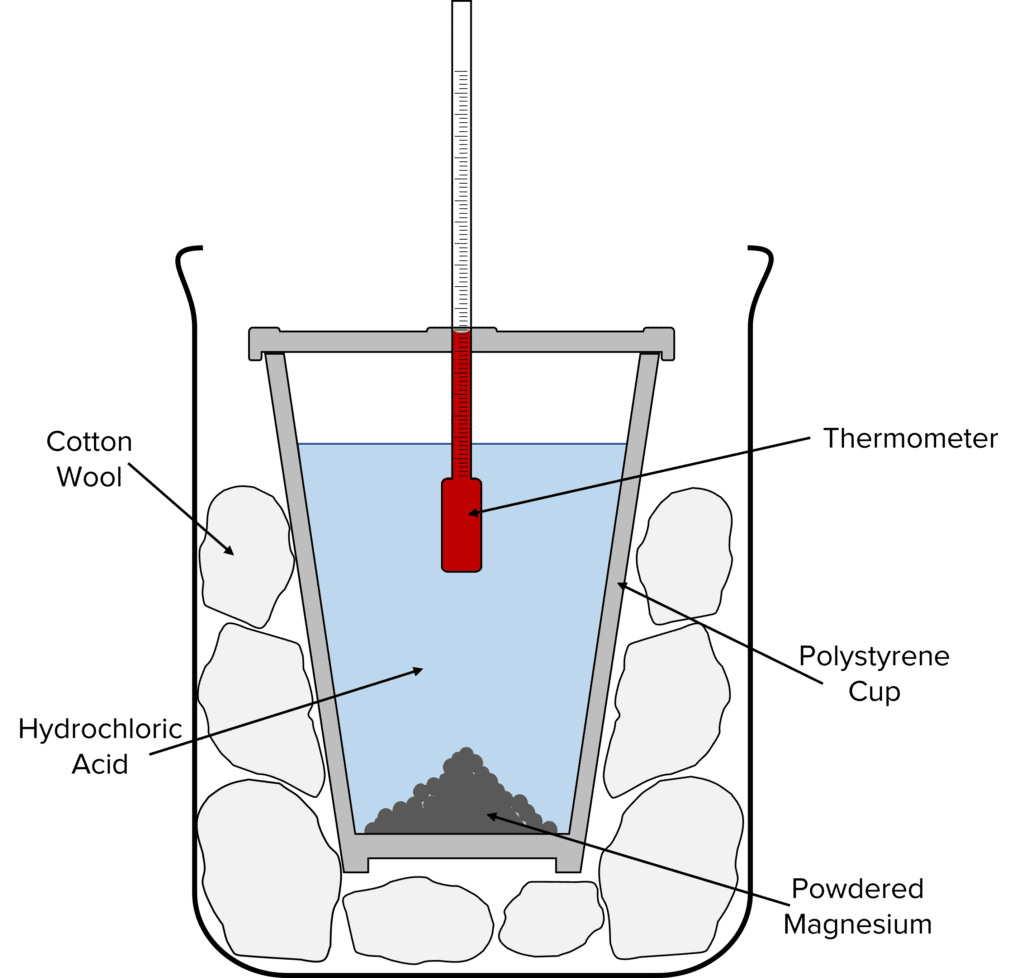
Method
- Prepare a sample of hydrochloric acid by heating 25\text{ cm}^{-3} to 25\degree \text{C}.
- Once heated, pour the sample into a polystyrene cup.
- Add 1\text{ g} of powdered magnesium to the cup.
- Place a lid over the cup with a thermometer pushed through the top. Make sure that the bulb of the thermometer is in the reaction mixture.
- Place the cup into a beaker full of cotton wool and measure the temperature every 30 seconds for 5 minutes and record the highest temperature.
- Repeat steps 1 to 5, increasing the concentration of acid by 0.2\text{ g dm}^{-3} at a time from 0.2\text{ g dm}^{-3} to 1.0\text{ g dm}^{-3}. Compare how changing the concentration of a reactant effects the energy change of the reaction.
Example: Calculating Overall Energy Change
Use the data in the table below to calculate the overall energy change of the following reaction.
\text{CH}_4+2\text{O}_2\rarr\text{CO}_2+2\text{H}_2\text{O}
| Bond | \text{C—H} | \text{O = O} | \text{C = O} | \text{O—H} |
| Bond Energy/\text{ kJ mol}^{-1} | \textcolor{#00bfa8}{413} | \textcolor{#f21cc2}{495} | \textcolor{#a233ff}{799} | \textcolor{#eb6517}{467} |
[3 marks]
Step 1: Count the number of bonds made and broken:
| Bond | \text{C—H} | \text{O = O} | \text{C = O} | \text{O—H} |
| Number Broken/ Made | 4 | 2 | 2 | 4 |
Step 2: Calculate energy change of the reaction.
\begin{aligned}\text{Energy Change}&=\left(\left[4\times\textcolor{#00bfa8}{413}\right]+\left[2\times\textcolor{#f21cc2}{495}\right]\right)-\left(\left[2\times\textcolor{#a233ff}{799}\right]+\left[4\times\textcolor{#eb6517}{467}\right]\right)\\ &=\left(1652+990\right)-\left(1598+1868\right)\\ &=2642-3466\\ &=\textcolor{#008d65}{-824\text{ kJ mol}^{-1}}\end{aligned}
As the change in energy is negative, we can deduce that energy is being transferred to the surroundings. As such, we can tell that the reaction is exothermic.
Endothermic and Exothermic Reactions Example Questions
Question 1: Define the term exothermic.
[2 marks]
A chemical reaction in which the energy of the reactants is higher than the products and that releases energy to the surroundings.
Question 2: State whether the profile below shows an endothermic or exothermic reaction. Explain your answer.
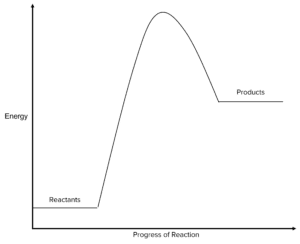
[3 marks]
The reaction is endothermic.
The reactants have a lower energy than the products. This means energy is absorbed by the reaction, making it endothermic.
Question 3: From the data below, calculate the overall energy change of the following reaction.
2\text{H}_2\text{O}\rarr2\text{H}_2+\text{O}_2
|
Bond |
\text{O—H} | \text{H—H} | \text{O = O} |
| Bond Energy /\text{ kJ mol}^{-1} | 467 | 432 | 495 |
[3 marks]
Step 1: Calculate the number of bonds broken and made.
|
Bond |
\text{O—H} | \text{H—H} | \text{O = O} |
| Number Broken/ Made | 4 | 2 | 1 |
Step 2: Calculate the overall energy change.
\begin{aligned}\text{Energy Change}&=\left(4\times467\right)-\left(\left[2\times432\right]+495\right)\\ &=1868-\left(864+495\right)\\ &=1868-1359\\ &=+509\text{ kJ mol}^{-1}\end{aligned}
(One mark for step 1. Four marks for step 2, one mark per correct line of working.)