Electrochemical Cells
Electrochemical Cells Revision
Electrochemical Cells
Electrochemical cells are a unique and useful application of a feature of chemical reactions called electrochemistry. Electrochemical cells make use of the fact that chemical reaction generate electricity. Electrochemical cells can be used to produce batteries, both rechargeable and non-rechargeable. Fuel cells are also an application of electrochemistry. They make use of oxygen to generate an electric current.
Electrochemical Cells and Voltage
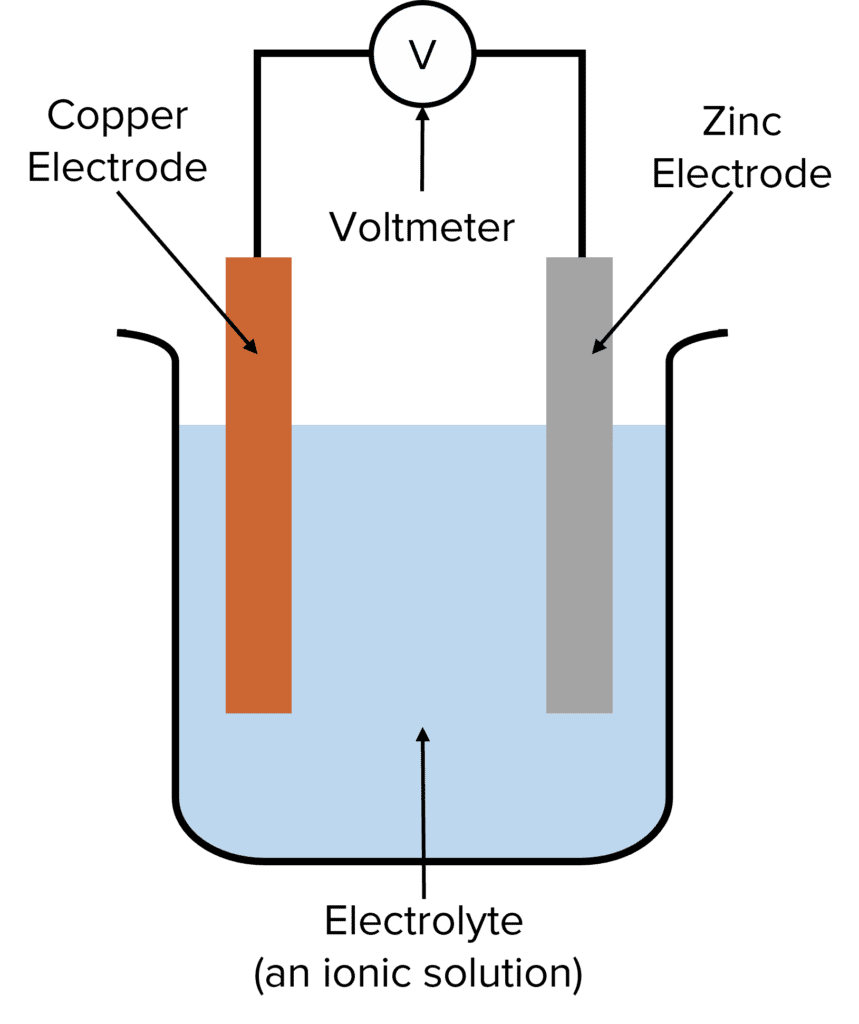
Electrochemical cells work in a manner that is almost identical to electrolysis except that, instead of using a voltage to generate chemical reactions, electrochemical cells use chemical reactions to generate voltage. To do this, electrochemical cells make use of the fact that chemical reactions generate electricity. The most basic electrochemical cells consist of two electrodes, one negative, one positive, immersed in an electrolyte (a solution that contains ions). The two electrodes are typically made of metal (such as copper, zinc, or platinum) as they need to be able to conduct electricity. The electrodes will react with the ions of the electrolyte, with these reactions generating the voltage of the cell.
The reactions at each electrode creates a charge difference between them. When the electrodes are connected by a wire, charge is able to flow between them. This generates an electrical current. The voltage produced by a cell can be measured by connecting a voltmeter to this wire.
The voltage of individual chemical cells is often very small (typically not more than 2 or 3\text{ V}). To generate a high enough voltage to be useful, multiple cells are required. Multiple cells can be connected together in series. This means that the cells are connected one after another, with a wire connecting the cells at the end of the line together. A group of cells connected this way in series is called a battery.
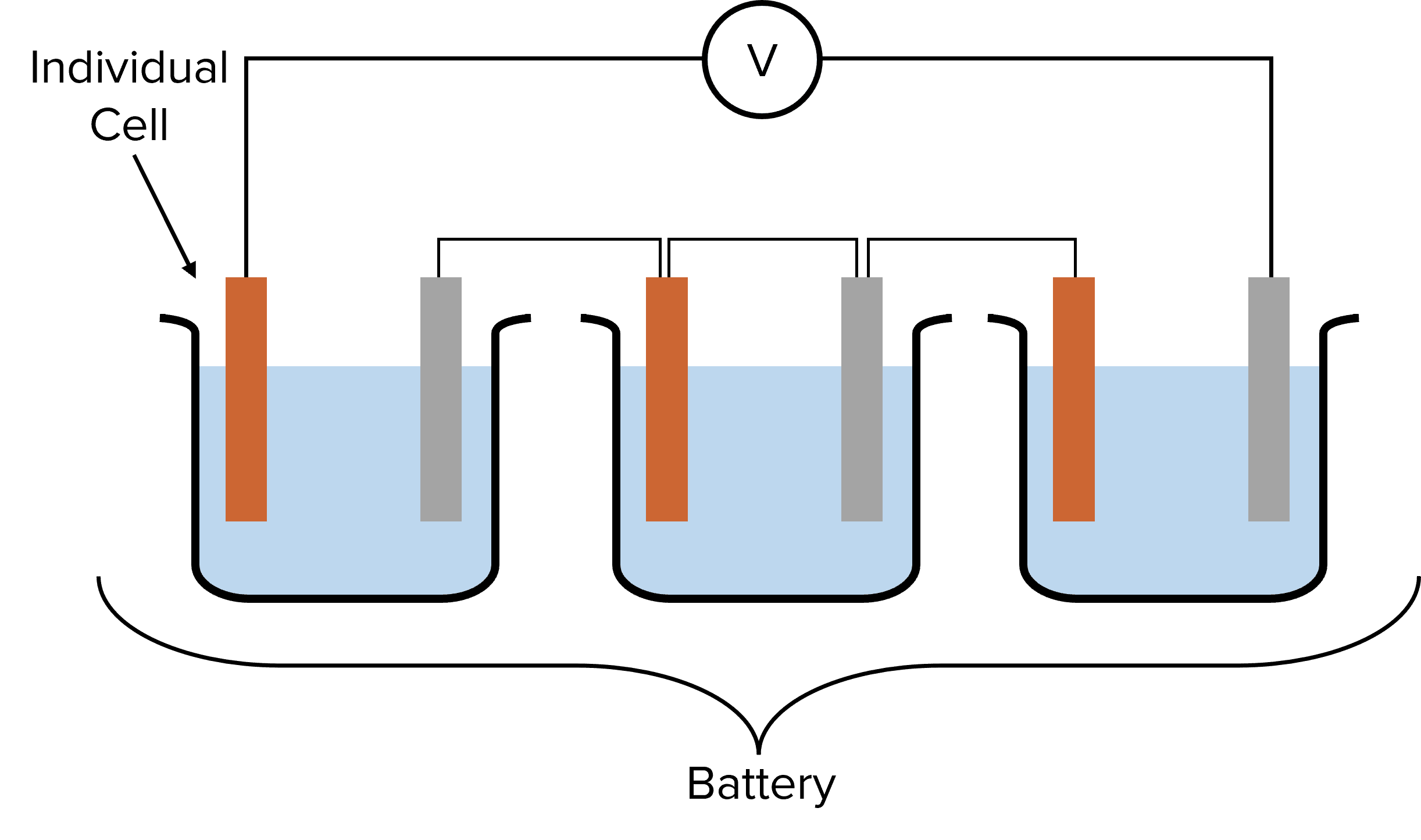
The voltage of a battery is calculated by adding up the voltages of the individual cells in the series. For example, if the cells in the diagram above have voltages of 2.3\text{ V}, 0.7\text{ V}, and 1.5\text{ V} respectively, then the overall voltage of the battery is:
\text{Battery Voltage}=2.3+0.7+1.5=4.5\text{ V}
A number of different factors will affect the voltage of an electrochemical cell. The reactivity of the different metals used as electrodes means that they will react differently with the same electrolyte. This difference in reactivity is what causes the charge difference between the electrodes. Cells that have electrodes that have a big difference in reactivity will generate a high voltage. Cells with electrodes of similar reactivity will have a lower voltage. If a cell was constructed using electrodes of the same metal there would be little to no current generated at all.
The electrolyte used will also affect the voltage of the cell, as different ions will react with the electrodes in different ways.
Rechargeable and Non-Rechargeable Batteries

Batteries constructed using electrochemical cells may be rechargeable or non-rechargeable, depending on the nature of the reactions taking place in the individual chemical cells.
In some chemical cells, the reactions that happen at the electrodes may be irreversible. This means that once the reactants of the chemical reaction are used up they cannot be recovered. The chemical reaction can only proceed in one direction. When any one of the reactants of an electrochemical cell is used up all reactions in that cell stop. At this point no more voltage is produced. When this happens to the cells of a battery, the battery stops producing electricity and needs to be replaced; it is non-rechargeable.
One example of a non-rechargeable battery is the common alkaline battery, used to power things like Christmas tree decorations or remote controls.
In rechargeable batteries, the chemical reactions taking place in the individual cells are reversible. This means that, by applying an external voltage, the chemical reactions can be driven in the opposite direction to the one when followed when generating current. This regenerates the original reactants from the products allowing it to produce a voltage once again. This process is known as recharging.
The most widespread example of a rechargeable battery is the lithium ion battery. These batteries are used to power most portable electronics such as phones, tablets, and even electric vehicles.
Fuel Cells
Fuel Cells are special types of electrochemical cell that require a constant supply of fuel to operate. One of the most widely used fuel cells is the hydrogen-oxygen fuel cell.
In the hydrogen-oxygen fuel cell, hydrogen and oxygen are fed into a cell and react to form water in the following overall reaction.
2\text{H}_2+\text{O}_2\rarr2\text{H}_2\text{O}
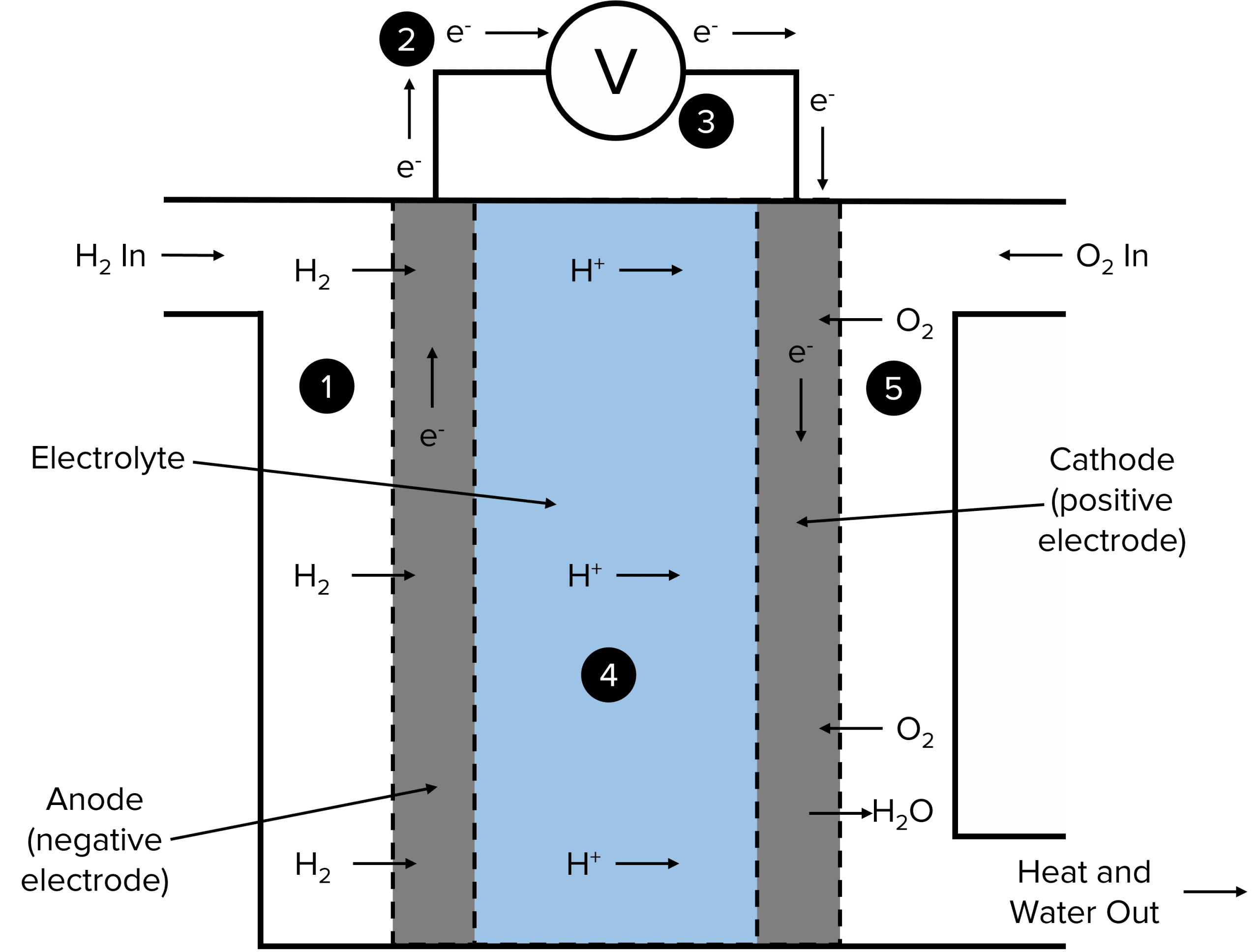
This reaction can be broken down into two redox half equations that take place at the anode and the cathode. At the anode (1), hydrogen gas is fed into the fuel cell and is oxidised to form \text{H}^{+} ions in the following half equation.
\text{H}_2\rarr2\text{H}^{+}+2\text{e}^{-}
The electrons that are donated by this process are then channeled into the external circuit (2), creating a current. This current can be measured using a voltmeter (3). The \text{H}^{+} ions then diffuse through the anode (usually a permeable carbon structure) and into the electrolyte (4).
At the cathode (5), oxygen gas is fed in and reduced by the electrons flowing in from the external circuit to form \text{O}^{2-} ions in the half equation:
\text{O}_2+4\text{e}^{-}\rarr2\text{O}^{2-}
These \text{O}^{2-} ions will the react with the \text{H}^{+} ions diffusing through the electrolyte from the anode to form \text{H}_2\text{O}. To construct the overall equation, the half equation for the oxidation of hydrogen must be multiplied by two. This gives an equal number of electrons on each side of the overall equation, allowing them to be canceled out.
Fuel cells have generally been seen as more environmentally friendly than both rechargeable and non-rechargeable batteries. As fuel cells work off a constant supply of hydrogen and oxygen, they do not have to be discarded as often as non-rechargeable cells, cutting down on chemical pollution. The components of fuel cells are also more easily obtained, with oxygen available from the air and hydrogen gas easily producible. The components in rechargeable cells are often less environmentally friendly to produce, with the precious metals necessary for some requiring large scale mining operations to produce.
The by-products of fuel cells are also less damaging to the environment than the components of tradition electrochemical cells. The hydrogen-oxygen fuel cell only produces water as a waste product.
The fuel cell does have drawbacks however. The requirement of a constant supply of hydrogen gas means this gas must be stored. Hydrogen is a highly flammable gas and as such storing it in large quantities poses a risk of explosion. As it is a gas, it also requires a large amount of space for storage.
Hydrogen-oxygen fuel cells have seen a variety of uses, including as power supplies onboard space craft such as the Space Shuttle and the international space station. There is also the potential for hydrogen-oxygen fuel cells to provide the power needed for electric vehicles.
Electrochemical Cells Example Questions
Question 1: State the main components of an electrochemical cells.
[3 marks]
- A positive electrode.
- A negative electrode.
- An electrolyte.
Question 2: Calculate the overall voltage of the battery shown below.
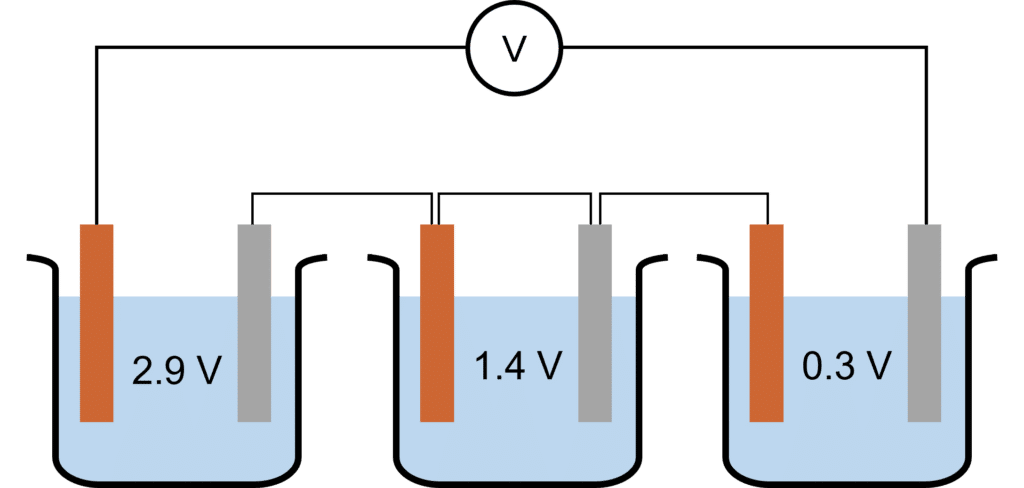
[2 marks]
\begin{aligned}\text{Battery Voltage}&=2.9+1.4+0.3\\ &=4.6\text{ V}\end{aligned}
Question 3: Give an example and a use of a rechargeable cell.
[2 marks]
Example: Lithium ion battery.
Uses: Powering portable electronics.
Question 4: Give the half equations for the anode and cathode reactions in the hydrogen-oxygen fuel cell.
\text{Anode Half Equation: }\text{H}_2+2\text{e}^{-}\rarr2\text{H}^{+}
\text{Cathode Half Equation: }\text{O}_2+4\text{e}^{-}\rarr2\text{O}^{2-}