Metals
Metals Revision
Metals
Metals are the most common elements on Earth. Fully 78\% of the elements on the periodic table are metals. With this volume, comes wide range of properties and chemical behaviours. That does not mean however that there are no similarities. All metals, for instance, can form oxides, can conduct electricity, and, with the exception of mercury, are solids at room temperature. The reactivity of metals is governed by their desire to form positive ions. This leads metals to react readily with oxygen and even more so with water. The reactivity of metals can be compared using the reactivity series.
Reactivity of Metals
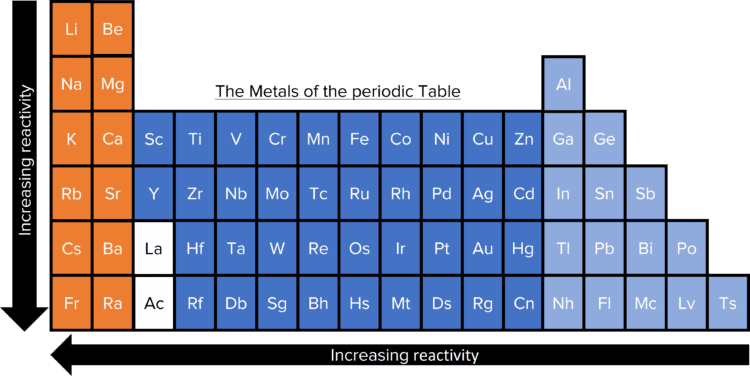
Across the periodic table, the reactivity of metals of increases from the top of a group to the bottom and from the right of a period to the left. Cesium (Cs) is them most reactive naturally occurring metal, though the laboratory produced element francium (Fr) is the most reactive overall. The least reactive of the metal elements is platinum (Pt). This overall trend can be seen in some of the individual groups of the periodic table. It is most prominent in groups 1 and 2. Here there is a strong increase in the reactivity of the elements as we go down the group from lithium and beryllium to francium and radium. This reactivity can be considered in terms of the ability of the metal to form positive ions in reactions. The more reactive a metal, the easier it is for it to lose electrons to become positive.
Reactivity of Metals with Acids and Water
The reactivity of metals can be investigated by studying their reactions with acids and water.
When reacted with an acid, a metal will produce a (usually) solid salt and hydrogen gas:
\text{Metal} + \text{Acid} \rarr \text{Salt} + \text{Hydrogen}
When reacted with water, metals will produce a metal hydroxide and hydrogen gas:
\text{Metal} + \text{Water} \rarr \text{Metal Hydroxide} + \text{Hydrogen}
The reactivity of a metal will determine how quickly a metal will react in water or acid, and under what conditions. More reactive metals, such as sodium or magnesium, will react readily (even violently) with dilute acids; less reactive metals will react only very slowly.
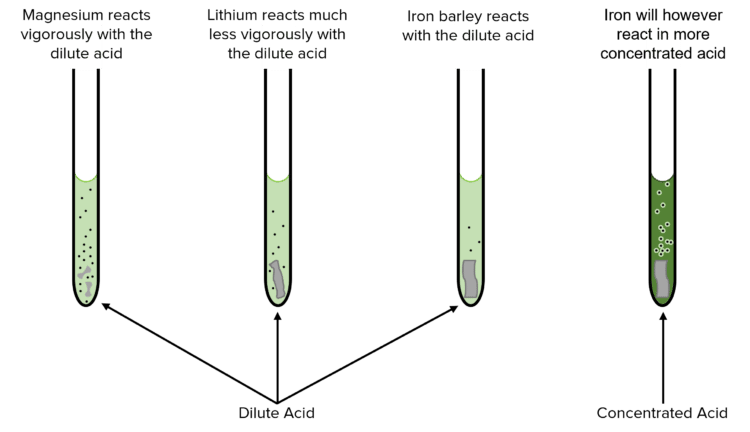
Similarly, more reactive metals will react readily with cold water where as less reactive ones will not react.
The Reactivity Series
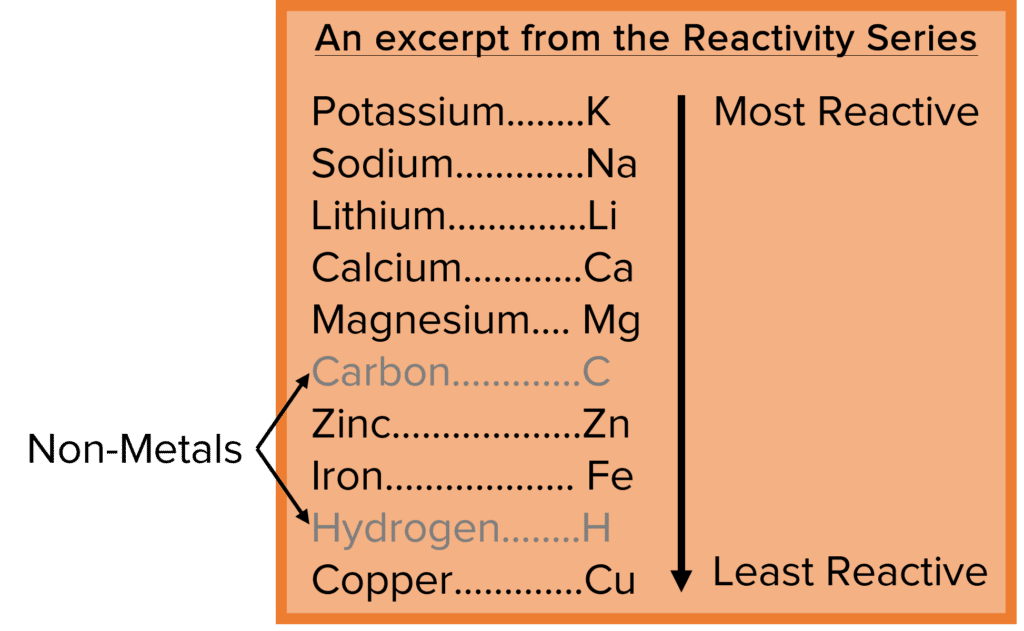
By carrying out these reactions with lots of different elements, using lots of different conditions, scientists have been able put the metals in order of their reactivity. This list is know as the reactivity series and it lists the metals from the most to the least reactive. The higher a metal is found in the reactivity series, the more readily it will react with acids and water. The lower a metal is found, the less readily it will react.
Though composed primarily of metals, the reactivity series does contain some non-metal elements, for example carbon and hydrogen. The presence of these non-metal elements can tell us about the different methods that can be used to obtain pure metals from their ores.
The Extraction of Metals from their Ores
It is fairly uncommon for metals to occur naturally in their pure elemental form. Instead, metals tend to appear in the Earth’s crust as compounds known as ores. These compounds are often (but far from exclusively) metal oxides. Metal oxide ores are formed by the reaction of elemental metals with oxygen in a process called oxidation as the metals are gaining oxygen atoms.
\text{Metal}+\text{Oxygen}\rarr\text{Metal Oxide}
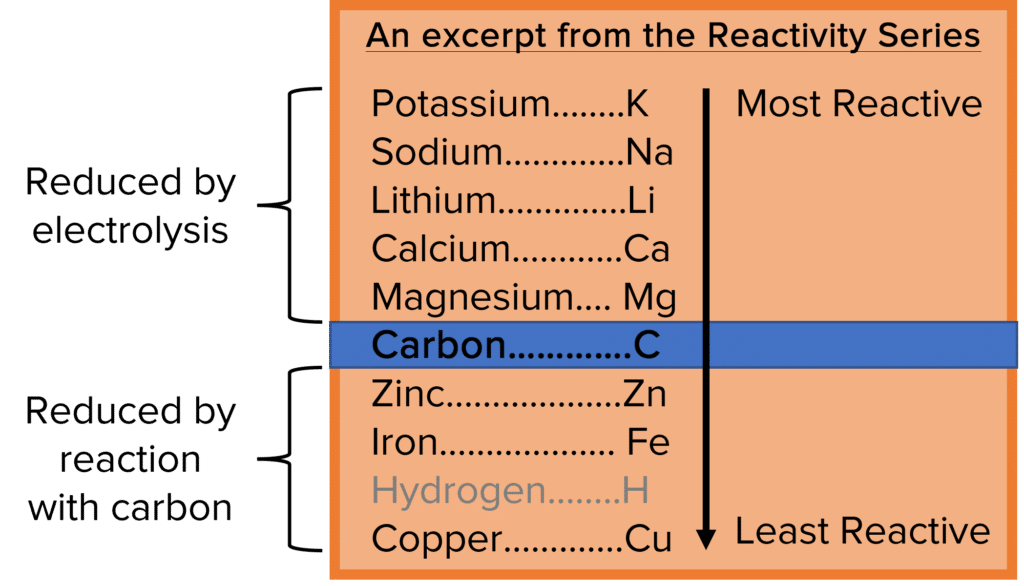
To extract pure metals from their oxide ores, they must be reduced (i.e. they must lose their oxygen atoms). This is typically done in one of two ways:
- Reduction by electrolysis
- Reduction by reaction with carbon
Which method is used will depend on the position of the metal in the reactivity series relative to that of carbon. Metals that come higher in the reactivity series and so are more reactive than carbon will be reduced using electrolysis. Those lower in the reactivity series and so are less reactive will be reduced by reaction with carbon. This is because metal oxides will not lose their oxygen when the metal is more reactive than carbon.
Reduction by carbon is a preferable method of extraction, as this is much less expensive than extraction using electrolysis. One common example of this method is the the reduction of iron (III) oxide to produce pure iron. In this process (known as smelting) iron (III) oxide is heated to a very high temperature (above 1200\degree \text{C}) in blast furnace. Carbon is added and a reaction takes place to form pure iron:
2\text{Fe}_2\text{O}_3 + 3\text{C} \rarr 4\text{Fe} + 3\text{CO}_2
In some cases, such as those of gold and platinum, a metal may be so unreactive that it does not naturally form ores. In these cases, the metal can be found in its pure elemental form.
Oxidation and Reduction
The extraction of metals from their ores are just one example of a family of chemical reactions known as redox reactions. Redox reactions are ones that involve the transfer of electrons between the reactants. The name is a combination of the two processes that take place in a redox reaction:
- Reduction: The gain of electrons by an atom or ion and thus a reduction in its charge.
- Oxidation: The loss of electrons by an atom or ion and thus an increase in its charge.
The term oxidation comes from the early understanding of these reactions involving the gain of oxygen atoms by a reactant to form an oxide. Later, as scientists began to understand more about the chemicals processes involved, the definition was changed to a more accurate one.
Redox reactions can be identified by considering the oxidation states of the elements involved. If there is a change in these across the reaction, then it is a redox process.
Take the example of the reaction between solid sodium and chlorine:
2\text{Na} + \text{Cl}_2 \rarr 2\text{NaCl}
Much like balancing a reaction, we can tally the oxidation states of the reactants and products to see if there has been a change in oxidation state:
| Right Hand Side | Left Hand Side | |||
| Element | \text{Na} | \text{Cl} | \text{Na} | \text{Cl} |
| Oxidation State | 0 | 0 | +1 | -1 |
Both reactants start out with an oxidation state of 0 (i.e. they have no charge). After the reaction, sodium has lost 1 electron to gain a +1 charge. Chlorine has gained 1 electron to gain a -1 charge. As both elements have undergone a change in their oxidation state, we can identify the reaction as a redox reaction.
Example: Redox Reactions
Deduce if the reaction between ethanoic acid \left(\text{CH}_3\text{COOH}\right) and sodium hydroxide \text{NaOH} is a redox reaction:
\text{CH}_3\text{COOH}+\text{NaOH}\rarr\text{CH}_3\text{COONa}+\text{H}_2\text{O}
Hint: Consider ethanoic acid as an ionic compound made up of one \text{CH}_3\text{COO}^- ion and one \text{H}^+ ion.
[4 marks]
Step 1: Tally the oxidation states:
| Left hand Side | Right Hand Side | |||||||
| Compound | \text{CH}_3\text{COO} | \text{H} | \text{Na} | \text{O} | \text{CH}_3\text{COO} | \text{H} | \text{Na} | \text{O} |
| Oxidation State | -1 | +1 | +1 | -2 | -1 | +1 | +1 | -2 |
Step 2: Deduce whether or not the reaction is a redox reaction.
The oxidations states of all the species in the reaction are the same on both the right and left hand sides. As such there is no change in oxidation state across the reaction. This means that the reaction is not a redox reaction.
Metals Example Questions
Question 1: Rank the following metals in order of increasing reactivity.
- Sodium
- Lithium
- Cesium
- Magnesium
[1 mark]
In order of increasing reactivity:
Magnesium < Lithium < Sodium < Cesium
Question 2: Suggest, with reasons, extraction methods for sodium and copper from their respective metal oxide ores.
[4 marks]
Sodium should be extracted using electrolysis. This is because its above carbon in the reactivity series and therefore more reactive.
Copper should be extracted by reduction by carbon. This is because copper is below carbon in the reactivity series and therefore less reactive.
Question 3: Identify the following statements as either true or false:
- Calcium can be extracted from its metal oxide ore with reduction by carbon.
- Oxidation is the loss of oxygen atoms.
- All metals can conduct electricity.
[3 marks]
- False – Calcium is more reactive than carbon so it can’t reduce calcium oxide.
- False – Oxidation is the gain of oxygen atoms.
- True – Metals conduct electricity thanks to the “sea” of delocalized electrons in their structure.
Question 4: An unknown metal is added to a test tube containing a solution of dilute hydrochloric acid. No reaction is observed.
A sample of the same unknown metal is added to a different test tube containing a much more concentrated solution of hydrochloric acid. In this case a reaction is observed.
State which of the following two metals is the unknown sample more likely to be. Explain your reasoning.
- Copper
- Potassium
[4 marks]
The unknown metal is likely to be A (copper).
Copper is low down in the reactivity series and so is relatively unreactive. This means it will not react with dilute hydrochloric acid, only concentrated hydrochloric acid.
Sodium is higher in the reactivity series and so would react with both.
Question 5: Determine whether or not the following reaction is a redox reaction:
\text{NaHCO}_3+\text{NaCl}\rarr\text{NaCl}+\text{H}_2\text{O}+\text{CO}_2
[5 marks]
The best way to determine if a reaction is redox is to compare the oxidation states of the elements on both sides of the reaction:
| Left Hand Side | Right Hand Side | |||||||||
| Element | Na | H | C | O | Cl | Na | H | C | O | Cl |
| Oxidation state | +1 | +1 | +4 | -2 | -1 | +1 | +1 | +4 | -2 | -1 |
All the elements retain their oxidation states through the reaction. This means that the reaction is not a redox reaction.
(2 marks for correct oxidation states on the LHS. 2 marks for correct oxidation states on the RHS. 1 mark for correct determination)






