Metallic Bonding
Metallic Bonding Revision
Metallic Bonding
Metallic bonding occurs when atoms of a metal elements bond with themselves. Metallic bonding operates in a similar way to ionic bonding, relying on electrostatic forces of attraction to keep the metal together. However, there are no negative ions in a metallic bond, instead attraction is between positive metal ions and negative electrons.
The Structures of Metals
Metals, like ionic substances, exist in a lattice when solid and, like ionic solids and giant covalent structures, contain millions of atoms. However, unlike the lattice of an ionic solid, metal lattices do not alternate between positive and negative ions. Instead, metals exist as a lattice of positive metal ions, surrounded by a “sea” of delocalised electrons. This sea of electrons is created when metal atoms come together and all give up their outer shell electrons to form a shared group between them. The now positively charged metal ions are attracted to the sea of electrons through strong electrostatic forces. This resulting attraction is what we call metallic bonding.
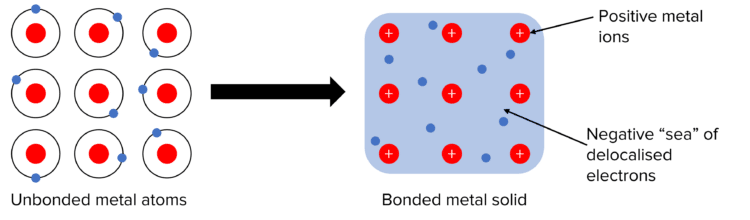
Properties of Metals
The properties of metals are a result of the forces of electrostatic attraction between the positive metal lattice, and negative “sea” of delocalised electrons. This electrostatic attraction is very strong, and requires a lot of energy to over come. As a result, metals typically have high melting points (often ranging from 400\degree \text{C} to over 3000\degree \text{C}) There are some exceptions to this trend however. Mercury has the lowest melting point of all the metals, all the way down at \text{-}39\degree \text{C}.
Metals are also able to conduct electricity when solid. This is because the delocalised electrons in the metal structure are not fixed in place and can move around freely. This lets them carry an electric charge, even when the metal is in the solid state (unlike ionic compounds which can only conduct when molten or in solution). Electrons can also carry thermal energy through the solid, making metals good conductors of heat as well as electricity.
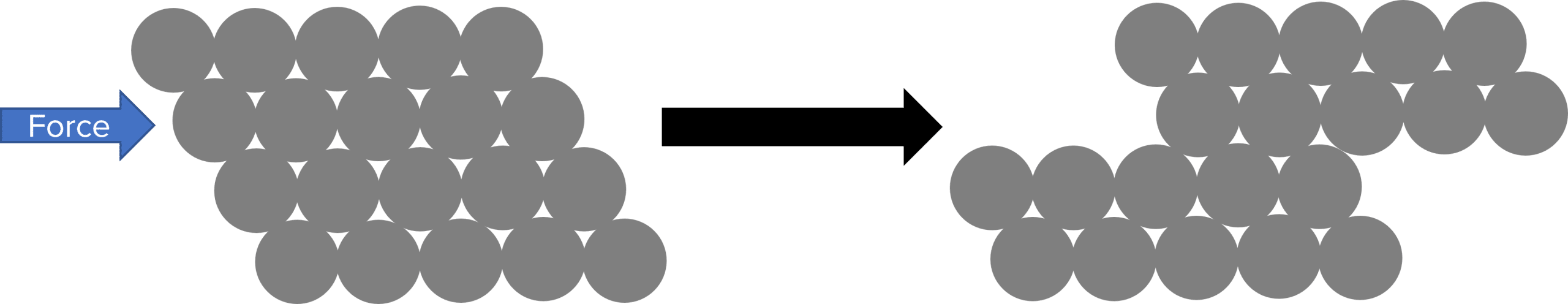
The lattice structure of metals means that they are also very ductile (they can be bent and formed into different shapes). Because the metal ions arranged in layers, they are able to slide over one another if a force is applied. This sliding will change the overall shape of the metal, allowing it to be shaped.
Unlike ionic solids, metals are not typically soluble in water. This is because the attraction between the positive metal lattice and negative “sea” of electrons is much stronger than that between the ions and water.
Metallic Bonding Example Questions
Question 1: Compare and contrast the bonding in ionic and metallic structures.
[4 marks]
Any 4 from the below points.
- Both structures contain lattices.
- Both structures rely on strong electrostatic attractions.
- Both structures have high melting points.
- Both structures can conduct in the liquid phase.
- Metals can conduct when solid, ionic solids can not.
- Ionic solids are typically soluble in water, metals are not.
Question 2: A student carries out a test to find the melting points of a range of substances. There results were:
| \text{Substance} | A | B | C | D | E |
| \text{Melting Point}\degree \text{C} | 0 | \text{-}7 | \text{-}114 | 3414 | 112 |
Which substance is most likely to be a metal and why?
[2 marks]
Substance D
Metals have high melting points and substance D has a significantly higher melting point than the other substances tested.
Question 3: What is meant by the term ‘the “sea” of delocalised electrons’?
[1 mark]
The “sea” of delocalised electrons is the result of metal atoms coming together and each donating their outer electrons into a shared group.
Metallic Bonding Worksheet and Example Questions
Chemical Bonding: Metallic Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



