States of Matter
States of Matter Revision
States of Matter
Almost all of the matter we interact with is present in one of three distinct forms. These forms are solids (e.g. a lump of coal), liquids (e.g. water), and gases (e.g. air). Which form a given substance of matter exists in will depend on a range of different things. The most important of which are the temperature of the substance, the pressure applied to it, and the types of molecules that it is made up of. Each of the three states of matter have very different properties and behaviours.
The three states of matter can be described by particle theory, in which the particles (i.e. atoms, ions, molecules) of a substance are represented by hard, inelastic, solid spheres.
Solids
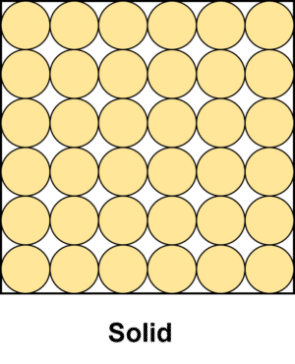
Solid matter is by far the most common state of matter found on earth (99.98\% of the planet’s mass). In solids, the particles or atoms of a substance are packed tightly together and vibrate about fixed positions. The vast majority of pure elements will exist as solids at room temperature. In terms of particle theory, solids can be described as spheres arranged so that they are in contact with one another, in an orderly pattern.
These particles are held together by strong forces of attraction. As a result of these strong forces, solids will have a fixed shape and volume (e.g. a square block of wood will stay square no matter its container).
At low enough temperatures, almost all substances will be solids. They are the lowest energy form of matter.
Liquids
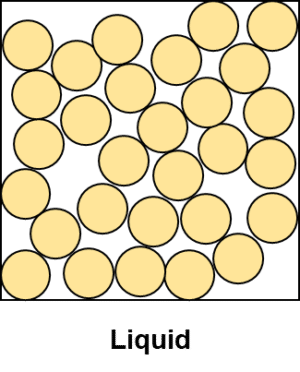
Liquids are made up of randomly arranged particles of a substances that are, unlike the particles in a solid, free to move around. This allows liquids to flow and to change their shape according to their container. Their volume however remains fixed. In terms of particle theory, liquids can be described as particles that have no orderly arrangement, where each particles is touching only a few others.
Though free to move, the particles in a liquid still remain close together. Though weaker than the forces in a solid, the forces between particles in a liquid still hold them into close groups.
Liquids are by far the least common form of matter for pure elements, with only two liquid elements at room temperature.
Gases
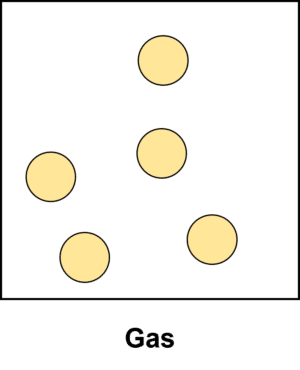
Gases are the least orderly of all the states of matter. In gases, the forces between particles are very weak and so they are able to move around completely freely and to spread out. As a result of this, gases are mostly empty space, with large distances between particles. Gases will always expand to fill whatever container they are in. In terms of particle theory, gases can be described as an arrangement of particles with no order, where none of the particles are in contact with one another.
The particles in a gas will move around randomly often bumping into one another.
Gases are the highest energy state of matter and the second most common state for pure elements.
Changing State
At a constant temperature and pressure, a substance will remain in its current state. However, if the temperature or pressure changes, the state of the substance may also change. There are four main ways in which a substance can change states; Melting, Boiling, Condensing, and Freezing.
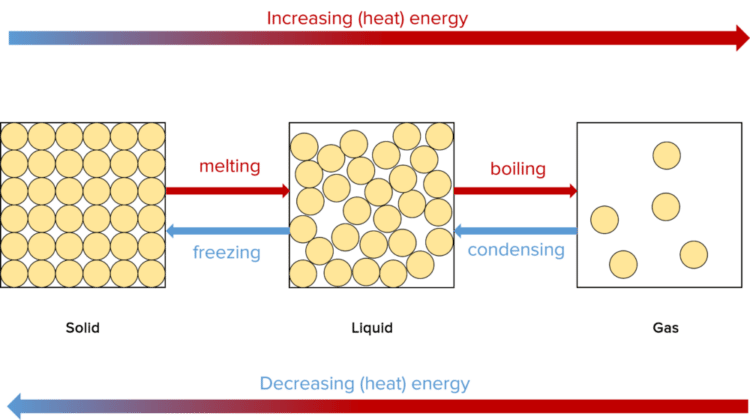
Melting:
Melting occurs when a solid is heated up to its melting point (the temperature at which a solid melts). At this temperature, the particles in the solid will have gained enough energy to overcome the forces between them.
Particles with stronger forces between them will have higher melting points (think metals and ionic compounds), those with weaker forces will have lower melting points (think ice, covalent compounds).
Once the particles have enough energy to overcome the forces between them, they break out of their fixed solid arrangement and begin to move around more freely. This greater freedom of movement marks the transition from the solid to liquid state.
Boiling:
Boiling occurs when a liquid is heated up to its boiling point (the temperature at which a liquid boils.) At this temperature, the particles in the liquid will have gained enough energy to overcome the remaining forces between them.
Once the particles have enough energy to overcome the forces between them, they will break away from each other, separating into individual particles. This greater freedom of movement and separation marks the transition from the liquid to the gaseous.
Condensation and freezing:
Condensation and freezing are the opposite processes to boiling and melting. In condensation, a gas cools down to the condensation point (equal to the boiling point of the compound) and looses energy, transitioning from the gaseous state to the liquid state. In freezing, a liquid cools down to its freezing point (equal to the melting point) as it loses energy, transitioning from the liquid state to the solid state.
Limitations of Particle Theory
Particle Theory can be very useful in helping to describe the states of matter. However, it has its limitations:
- Particle theory doesn’t consider the forces between molecules.
- It assumes that all particles are spheres, failing to account for the complex shapes of molecules.
- It assumes that all spheres are solid and inelastic, which does not reflect the true nature of atoms, ions, or molecules.
States of Matter Example Questions
Question 1: In which state of matter are the particles most closely held together?
[1 mark]
The solid state.
Question 2: The melting point of a silver is 961\degree \text{C}, and its boiling point is 2261\degree\text{C}. In which state of matter would silver be found if it was heated to 1200\degree \text{C}?
[2 marks]
1200\degree \text{C} is higher than the melting point of silver so it will be in the liquid state.
Question 3: In terms of particle theory, describe the difference between the solid and liquid states.
[4 marks]
- Solids are made up of an ordered arrangement particles.
- Particles in a solid are held very close together so that they are touching.
- Gasses are made up of completely disordered particles.
- The particles in gasses are very spread out. They do not touch.
Question 4: Name two limitations of particle theory.
[2 marks]
Any two from:
- Particle theory doesn’t consider the forces between molecules.
- It assumes that all particles are spheres, failing to account for the complex shapes of molecules.
- It assumes that all spheres are solid and inelastic, which does not reflect the true nature of atoms, ions, or molecules.
One mark per correct answer.