Ionic Bonding
Ionic Bonding Revision
Ions and Ionic Bonds
Ions are one of the most important types of particles in chemistry. Ions are atoms or molecules that have a charge. They gain this charge through either the loss or gain of one or more electrons. Ions of opposite charges can come together to form ionic bonds. These bonds are very strong and as such the resulting ionic compounds often have very high melting points. Ionic bonds happen between metal and non-metal elements (e.g. \text{Na} and \text{Cl} form \text{NaCl}, an ionic salt.)
Formation of Ions
Ions are formed when an atom gains or loses one or more electrons to form a charged particle. Whether or not an atom forms a positive or negative ion (i.e. whether it will lose or gain electrons) will depend on its electronic structure.
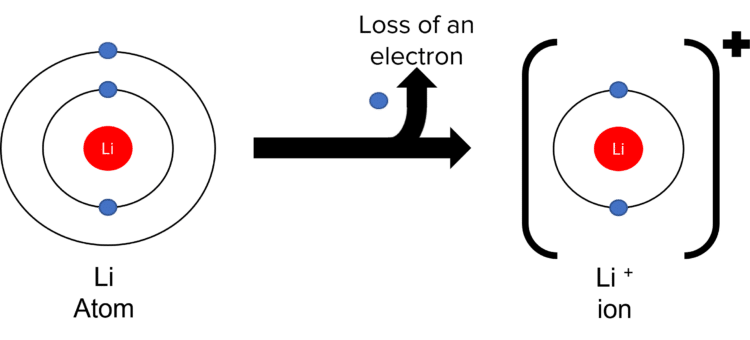
Positive ions are formed by the loss of electrons from less than half filled electron shells. Take as an example the formation of the \text{Li}^{+} ion.
- Lithium has an electronic structure of 2\text{,}1.
- This tells us that lithium has 2 electron shells, with 1 electron in the outermost shell.
- Li wants a full outer shell. The most efficient way to do this it for it to lose its outermost electron.
- The loss of the negatively charged electron leaves \text{Li} with a \text{+} 1 charge (number of positive protons – number of negative electrons = \text{+}1). This process is called ionisation.
Positive ions are almost always metals, as these elements tend to have outer shells that are less than half full. Common metal ions you may come across are \text{Na}^{+}, \text{Mg}^{2+}, and \text{Fe}^{3+}.
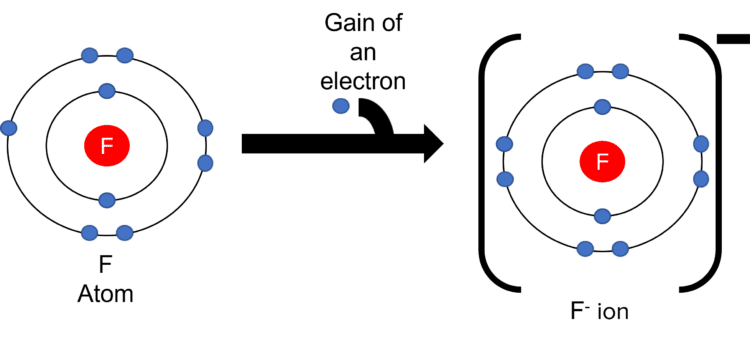
Negative ions are formed by the gain of electrons in more than half filled shells. Take for example the formation of the \text{F}^{-} ion.
- Fluorine has an electronic structure of 2\text{,}7.
- This tells us that fluorine has 2 electron shells, with 7 electrons in its outer most shell.
- Fluorine wants a full outer shell. The most efficient way to do this is for it to gain an electron.
- The gain of a negatively charged electron leaves \text{F} with a \text{-} 1 charge (number of positive protons – number of negative electrons = \text{-}1). This process is also called ionisation.
Negative ions are almost always non-metal elements, as these are the elements that tend to have outer shells that are more than half full. Common negative ions you may come across are \text{Cl}, and \text{O}^{2-}.
One quick way to work out the charge an atom, would have when ionised is to see what group it is in on the Periodic Table. Atoms in groups 1 and 2 will typically give \text{+} 1 and \text{+} 2 charges. Atoms in groups 6 and 7 will typically give \text{-} 1 and \text{-} 2 charges.
Ionic Bonds
Ionic bonds are formed when metal elements react with non-metal elements. To continue with the above example, consider the formation of the \text{LiF} molecule. When \text{Li} and \text{F} react, the lithium atom is able to lose its outer electron and give it to fluorine. This creates one positive \text{Li}^{+} ion and one negative \text{F}^{-} ion. Both ions will have full outer shells thanks to the transfer of lithium’s outer electron.
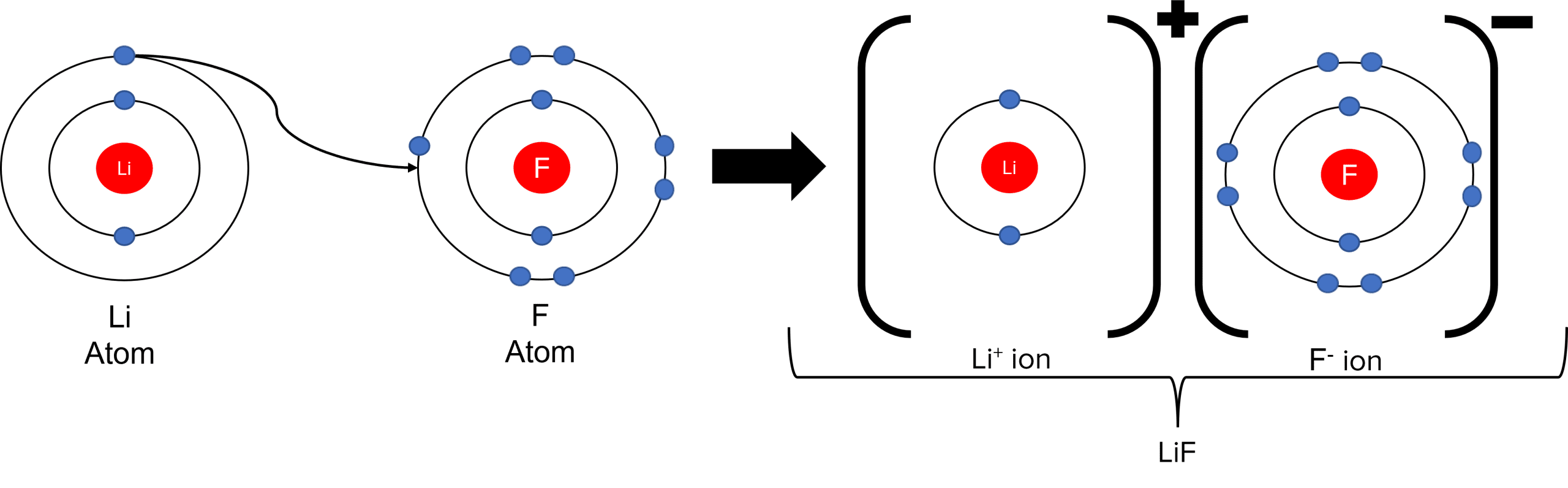
As the resulting ions are oppositely charged, there is a strong electrostatic attraction between them. This attraction will keep the two ions together in the form of an \text{LiF} molecule.
Ionic molecules will often have an overall charge of 0. This is because the charges in an ionic molecule will balance out. Using \text{LiF} as an example:
\text{Charge of LiF} = \text{Charge of Li ion} + \text{Charge of F ion}
\text{(+} 1 \text{) + (-} 1\text{) = } 0
When ions do not have charges of equal magnitude (e.g. the same value but opposite signs) then one or more of the ions will have to be multiplied to balance the overall charge of the molecule. Take the example of iron (III) chloride
\text{Charge Fe ion = +}3
\text{Charge Cl ion = -}1
0\text{ = (+}3\text{) - x(-}1\text{)} (where x is the represent the number of chloride ions)
\text{x = } \dfrac{3}{1}\text{ = } 3
This tells us that to have a balanced overall charge, we need to have 3\text{ Cl}^{-}ions for every 1\text{ Fe}^{+} ion. This gives a formula for iron (III) chloride of \text{FeCl}_3
Dot and Cross Diagrams
Ions and ionic molecules can be represented on paper through the use of dot and cross diagrams. In a dot and cross diagram, ions are drawn as a central nucleus, surrounded by rings of orbiting electrons (represented by either dots or crosses). The formation of ionic molecules can be shown by using dots to represent the electrons of one ion, and crosses for the electrons of another. By placing the dot of a metal ion in the outer ring of the crosses of a non-metal ion, we can show that an electron has been transferred:
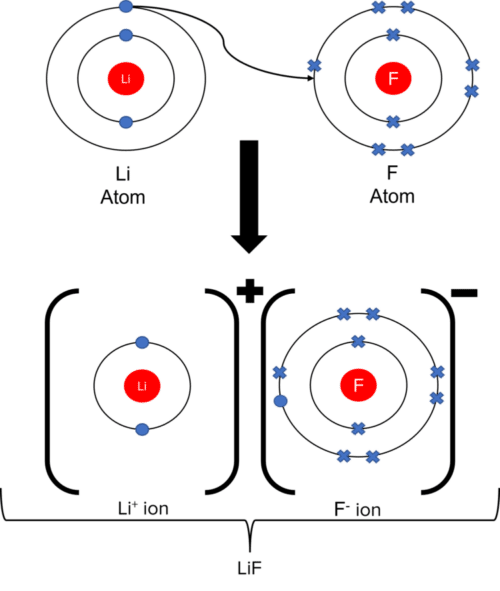
Ionic Structures and Their Properties
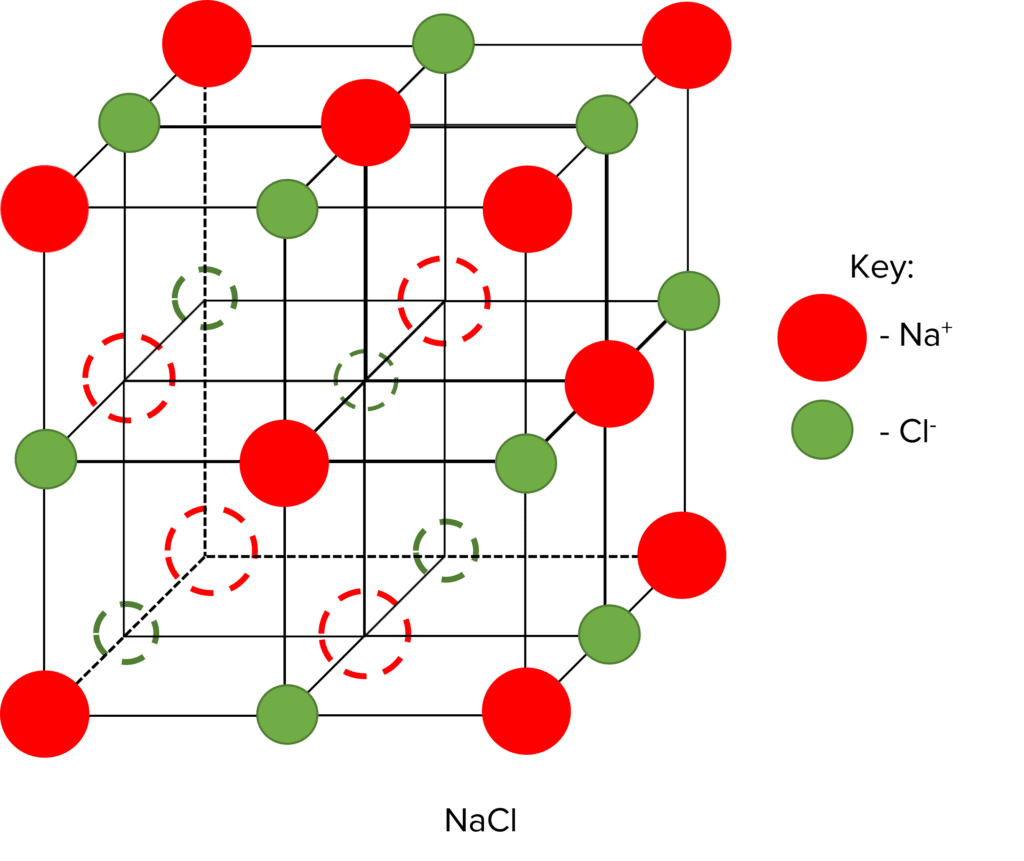
When ionic molecules come together to form solid compounds, they do so in a very orderly and defined way. Solid ionic compounds are made up of a giant ionic lattice. This name is very important, as each word tells you something about the structure of ionic solids.
- Giant: The solid structure is very large, containing millions of ions, held together by millions of bonds
- Ionic: The particles that make up the solid are ions, each with a negative or positive charge.
- Lattice: A lattice is an orderly structure made up of repeating patterns (or units). In the case of the ionic lattice, this repeating pattern is made up of alternating positive and negative ions.
Ionic solids tend to have incredibly high melting points (for example, the melting point of \text{NaCl} is 801\degree \text{C}). This is a result of the strong electrostatic attractions between oppositely charged ions in the lattice. These forces require a lot of energy to overcome.
Ionic solids are often easily soluble in water. This is because the electrostatic attractions between the ions in the lattice and the \text{H}_2\text{O} molecules is stronger than that between ions in the lattice. When in the solid state, ionic substances do not conduct electricity, this is because the ions in the lattice are fixed in place. Ionic substances will conduct when molten. This is because, in the liquid state, the ions are able to move around and as such carry a charge.
Ionic Bonding Example Questions
Question 1: Iron and chlorine react to form \text{FeCl}_{3}. In this reaction the iron atoms lose 3 electrons to form ions. What is the charge on these ions?
[1 mark]
Question 2: Iodine (group 7) reacts with potassium in solution to form potassium iodide (\text{KI}). What will be the charge of the iodide ion?
[1 mark]
Iodine is in group 7. Group 7 ions have a \text{-}1 charge.
Ion is \text{I}^{-}
Question 3: The melting point of strontium chloride (\text{SrCl}_{2}) is 874\degree \text{C}. Predict whether \text{SrCl}_{2} would conduct electricity at 630\degree \text{C}.
[2 marks]
Strontium chloride/\text{SrCl}_{2} will not conduct electricity at 630\degree \text{C}. This is because at this temperature \text{SrCl}_{2} will be solid and ionic compounds only conduct electricity when molten/ liquid (in solution).
Question 4: Sodium chloride (\text{NaCl}) and sulfuric acid (\text{H}_{2}\text{SO}_{4}) react to form sodium sulfate. Given that \text{Na} is a group 1 element, and the charge of a sulfate (\text{SO}_{4}) ion is \text{-}2, deduce the formula of sodium sulfate.
[4 marks]
- Sodium is in group 1 so its ion will have a \text{+}1 charge
- The overall charge of sodium sulfate/ an ionic molecule is 0
0\text{ = (-}2\text{) + x(+}1\text{)}
\text{x = }2
Therefore, the formula of sodium sulfate is: \text{Na}_{2}\text{SO}_{4}
(1 mark for correct calculation, 1 mark for correct formula)