Trends in the Periodic Table
Trends in the Periodic Table Revision
Trends in the Periodic Table
The Periodic Table groups elements of similar chemical properties, but there are still differences between the elements in these groups. Specifically, there are patterns in the chemical and physical properties of the elements within a group, and this is based on their electronic structure.
Metals and Non-Metals
Metals
The majority of the elements are metals, and they are found primarily on the left hand side of the periodic table. While there are specific patterns of chemical and physical properties in specific groups, metals on the whole tend to be shiny, hard, malleable (easily shaped), dense, good electrical and thermal conductors, and solids at room temperature. In chemical reactions, metal atoms typically gain a full outer shell by losing electrons.
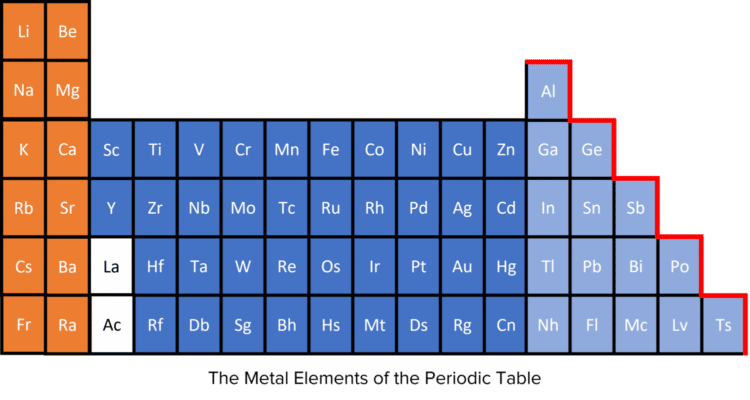
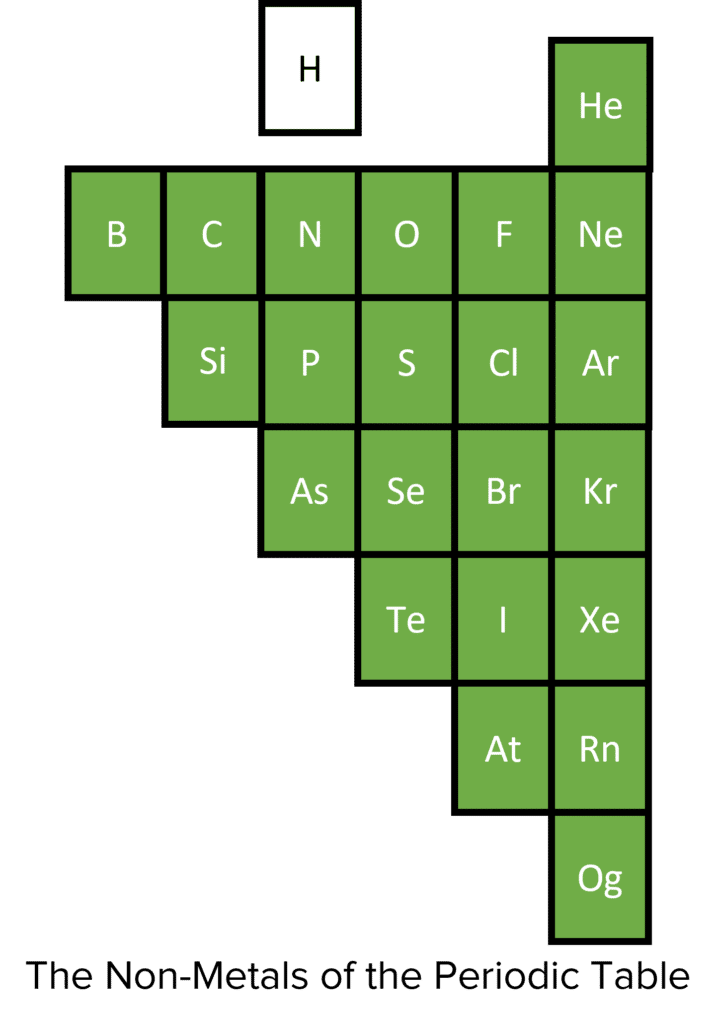
Non-Metals
There are far fewer non-metals, and they are found to the right of the periodic table. Non-metals also have specific patterns of chemical and physical properties within their groups, but generally tend to be dull, non-conductive, brittle, less dense (compared to metals) and gases/liquids at room temperature. In chemical reactions, non-metals typically gain a full outer shell by gaining or sharing electrons.
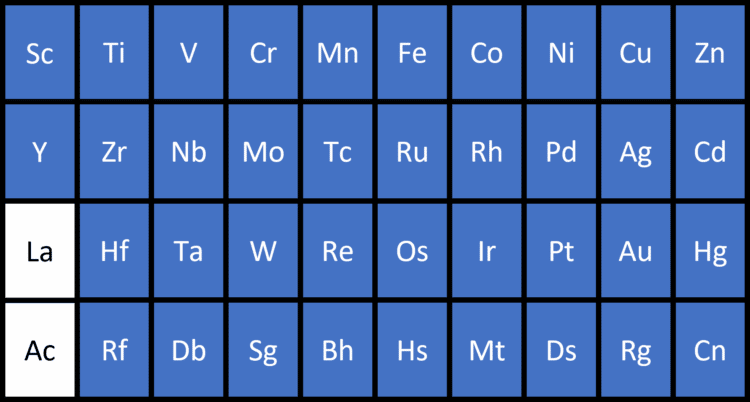
Transition Metals
The transition metals are a block of metals between groups 2 and 3 in the Periodic Table. They show all of the general properties of metals, but have useful additional properties. Specifically, they can form coloured compounds in chemical reactions, they can act as catalysts (substances which speed up chemical reactions without themselves being changed), and they can lose different numbers of electrons in different chemical reactions.
Group 1: Reactions and Trends
Group 1 elements are also known as the alkali metals, and they have similar chemical properties.
They react with water to produce metal hydroxide solutions (which are alkaline, hence the name of the group).
\text{Alkali Metal}+\text{Water}\rarr\text{Metal Hydroxide}+\text{Hydrogen}
\text{Sodium}+\text{Water}\rarr\text{Sodium Hydroxide}+\text{Hydrogen}
They react with acids to produce salts and hydrogen gas.
\text{Alkali Metal}+\text{Acid}\rarr\text{Salt}+\text{Hydrogen}
\text{Lithium}+\text{Sulfuric Acid}\rarr\text{Lithium Sulfate}+\text{Hydrogen}
They react with oxygen to produce metal oxides.
\text{Alkali Metal}+\text{Oxygen}\rarr\text{Metal Oxide}
\text{Potassium}+\text{Oxygen}\rarr\text{Potassium Oxide}
They react with halogens (group 7) to produce salts.
\text{Alkali Metal}+\text{Halogen}\rarr\text{Salt}
\text{Caesium}+\text{Chlorine}\rarr\text{Caesium Chloride}
The chemical reactivity (how vigorously they react) of the alkali metals increases down group 1. This means that sodium reacts more vigorously with water than lithium, and potassium reacts more vigorously with water than sodium.
When added to water, lithium will float on the surface. There will be mild effervescence (fizzing) whilst the reaction happens. Eventually all the lithium will have reacted and dissolve to form a lithium hydroxide solution.
When added to water, sodium reacts more vigorously. It will move around on the surface of the water and melt into a ball. There will be a much stronger effervescence.
When potassium is added to water, the reaction is more vigorous again. The potassium will melt into a ball and move around much more rapidly on the surface of the water. The effervescence will again be much stronger, and the heat of the reaction will be enough to ignite the hydrogen gas. The potassium will burn with a lilac flame.
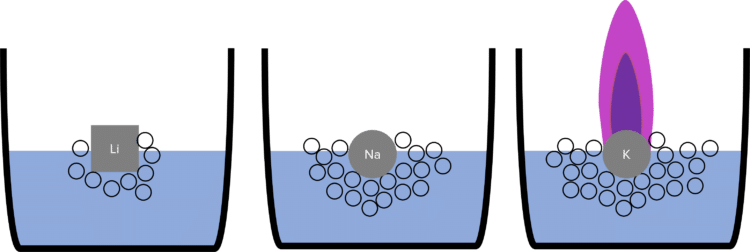
This is due to the electronic structure of the alkali metals. As they are in group 1, they each have a single electron in their outer shell. In chemical reactions, they lose this outer electron to gain a full outer shell. The more electron shells there are between the outer electron and the nucleus, the easier it is to lose the outer electron. (This is because the shells act as “shielding” from the positive charge of the nucleus). As the number of electron shells increases down group 1, the outer electron becomes easier to lose, and the reactivity of the elements increases.
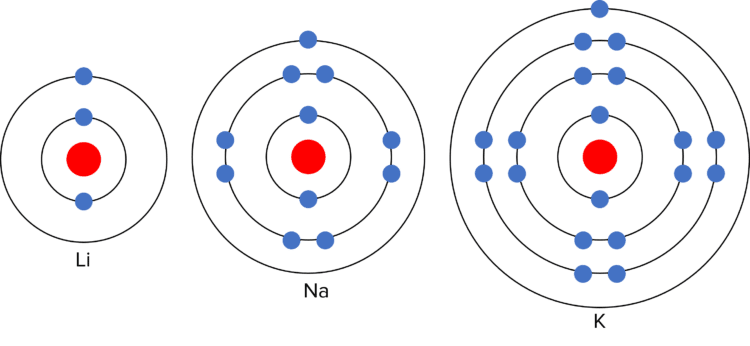
The alkali metals also have similar mechanical properties. They are shiny (when freshly cut), soft, low density (compared to other metals), and conduct heat and electricity. There is also a trend in the physical properties of the alkali metals. Down the group, the alkali metals show an increasing relative atomic mass, and decreasing melting and boiling points.
Group 7: Reactions and Trends
The group 7 elements are also known as the halogens. They are non-metals, and as elements they exist as two atoms chemically bonded together. The group 7 elements have similar chemical properties. They react with alkali metals to form salts.
\text{Halogen}+\text{Alkali Metal}\rarr\text{Salt}
\text{Chlorine}+\text{Rubidium}\rarr\text{Rubidium Chloride}
They can also undergo displacement reactions, where one halogen will displace another in a compound.
\text{Halogen}+\text{Compound}\rarr\text{Compound}+\text{(Displaced) Halogen}
\text{Chlorine}+\text{Sodium Bromide}\rarr\text{Sodium Chloride}+\text{Bromine}
The chemical reactivity (how vigorously they react) of the halogens decreases down group 7. This means that chlorine reacts less vigorously with sodium than fluorine, and bromine reacts less vigorously with sodium than chlorine. This is due to the electronic structure of the halogens. As they are in group 7, they each have seven electrons in their outer shell. In chemical reactions, they gain one additional electron to gain a full outer shell. The more electron shells there are between the outer electron and the nucleus, the harder it is to gain an additional outer electron. (This is because the shells act as “shielding” from the positive charge of the nucleus) As the number of electron shells increases down group 7, it becomes harder to gain the last outer electron, and the reactivity of the elements decreases.
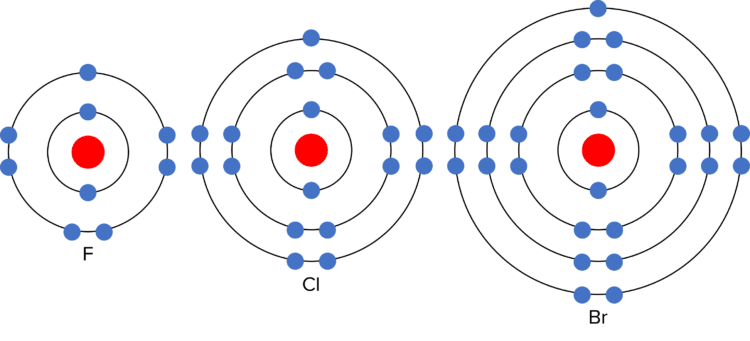
The halogens also have similar physical properties:
- They have a low density, and low melting and boiling points.
There is also a trend in the physical properties of the halogen.
- Down the group, the halogens show increasing relative atomic mass , and increasing melting and boiling points.
- At room temperature, Fluorine is a toxic yellow gas.
- At room temperature, Chlorine is a toxic, dense green gas.
- At room temperature, Bromine is a dense red-brown liquid.
- At room temperature, Iodine is a dark grey solid.
- When warmed above room temperature, Bromine will vaporise to form toxic red-brown bromine vapour, and iodine crystals will vaporise to form toxic purple iodine vapour.
Group 0: The Noble Gases
The elements in group 0 (also referred to as group 8) of the Periodic Table are called the noble gases. They are chemically inert, meaning they do not undertake chemical reactions with other atoms. As a result the noble gases exist as single atoms and do not bond with other atoms. They owe their inert nature to their electronic structure – they have full outer electron shells, and therefore do not lose or gain electrons in reactions.
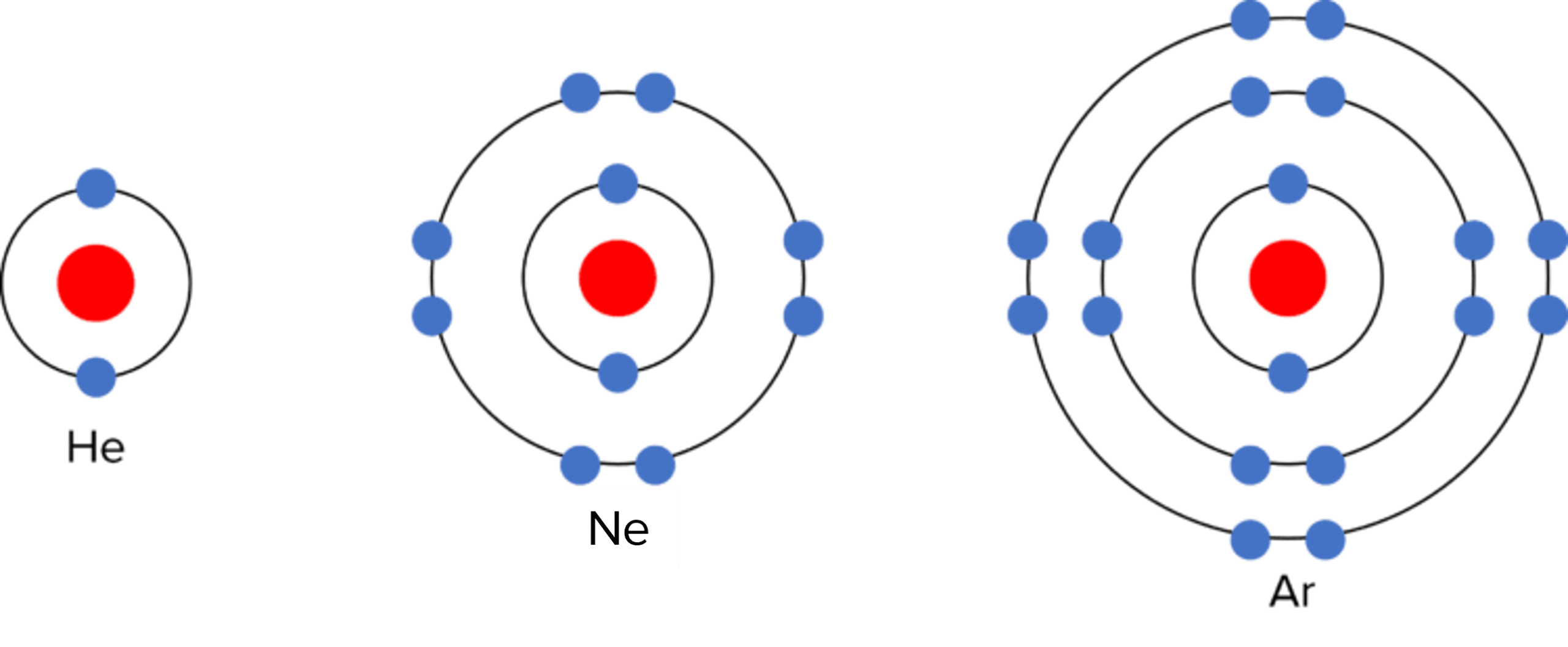
The noble gases also have similar physical properties. They have low densities, and low melting and boiling points. Down the group, the noble gases show a trend of increasing relative atomic mass, and increasing melting and boiling point.
Trends in the Periodic Table Example Questions
Question 1: Which side of the periodic table contains the metals?
[1 mark]
The left hand side.
Question 2: Give four properties of transition metals.
[4 marks]
Any four from:
- Hard
- Shiny
- Malleable
- Thermal/electrcal conductors
- Form coloured compounds
- Good catalysts
- Can lose different numbers of electrons in chemical reactions
Question 3: Describe and explain the trend in chemical reactivity in the group 1 alkali metals.
[5 marks]
- Reactivity increases down the group. Down the group the number of electron shells increases. This increases the “shielding” from the positive charge of the nucleus on the outer electron. This makes the outer electron easier to lose. This increases the reactivity of the group 1 elements which lose their outer electron in chemical reactions.
Question 4: Compare the trend in reactivity between the group 7 and group 0 elements.
[2 marks]
Group 7 elements decrease in reactivity down the group. Group 0 elements are all inert and show no trend in chemical reactivity down the group.
Question 5: Why are the group 0 elements chemically inert?
[1 mark]
They have full outer shells.
Trends in the Periodic Table Worksheet and Example Questions
The Periodic Table: Group Seven and Transition metals Questions
GCSEOfficial MMEThe Periodic Table: Group Zero and One Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now




