The Periodic Table
The Periodic Table Revision
The Periodic Table
Categorising and ordering substances helps us to understand and use them, and for many years there was debate about how to best categorise and order the elements. Two ideas stood out. The chemical properties of elements could be used to group similar elements. The atomic weight (essentially atomic mass, but without the basis of protons and neutrons) could be used to order the elements. But there were difficulties reconciling the two, and it took the work of Dmitri Mendeleev to give the basis for the modern Periodic Table.
Early Periodic Tables
In the 1800s there were around 60 known elements The majority of attempts to organise these elements focused on their atomic weight. When ordered in increasing atomic weight there emerged a roughly repeating pattern of similar chemical properties – more or less every 8 elements, the pattern of chemical reactivity repeated itself. This periodic repetition of properties is why the ordered elements are called a Periodic Table. However, this was an imperfect pattern, and when only atomic weight was used to organise the elements it led to groupings of elements that were dissimilar in their chemical properties.
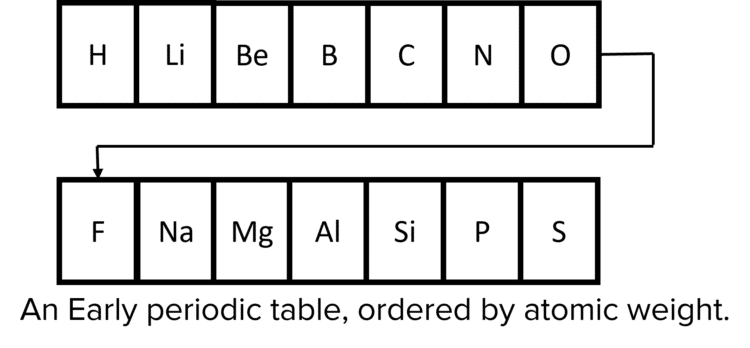
When scientists focused instead on the chemical properties of the known elements, they were able to group elements together more effectively, but this gave no insight into how their properties related to their atomic weights.
Dmitri Mendeleev
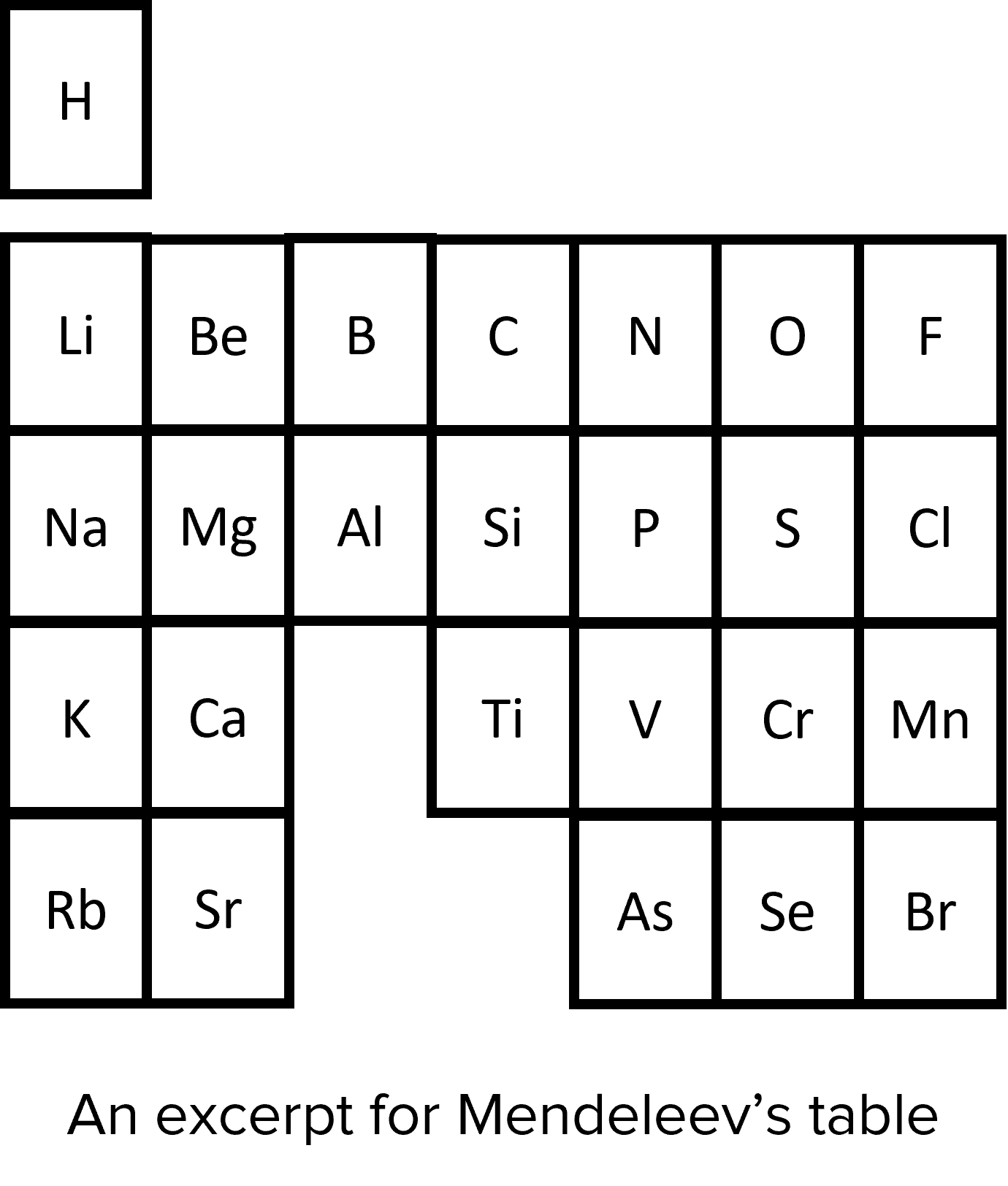
Dmitri Mendeleev was a Russian scientist working in the 1800s. He developed a way of categorising the known elements that took into consideration both atomic weight and chemical properties. He primarily ordered the elements by increasing atomic weight. However, when chemical properties suggested that elements should be grouped differently, Mendeleev relaxed the ordering by atomic weight so that as far as possible chemically similar elements were in the same group. Mendeleev also realised that some elements had not yet been discovered. He left gaps in his table to accommodate newly discovered elements, and used the properties of the elements around the gaps to predict the properties that the discovered elements would have. In later years, Mendeleev’s predictions proved to be very accurate. So much so that his work was accepted by the wider scientific community and became the basis of the modern periodic table.
The Modern Periodic Table
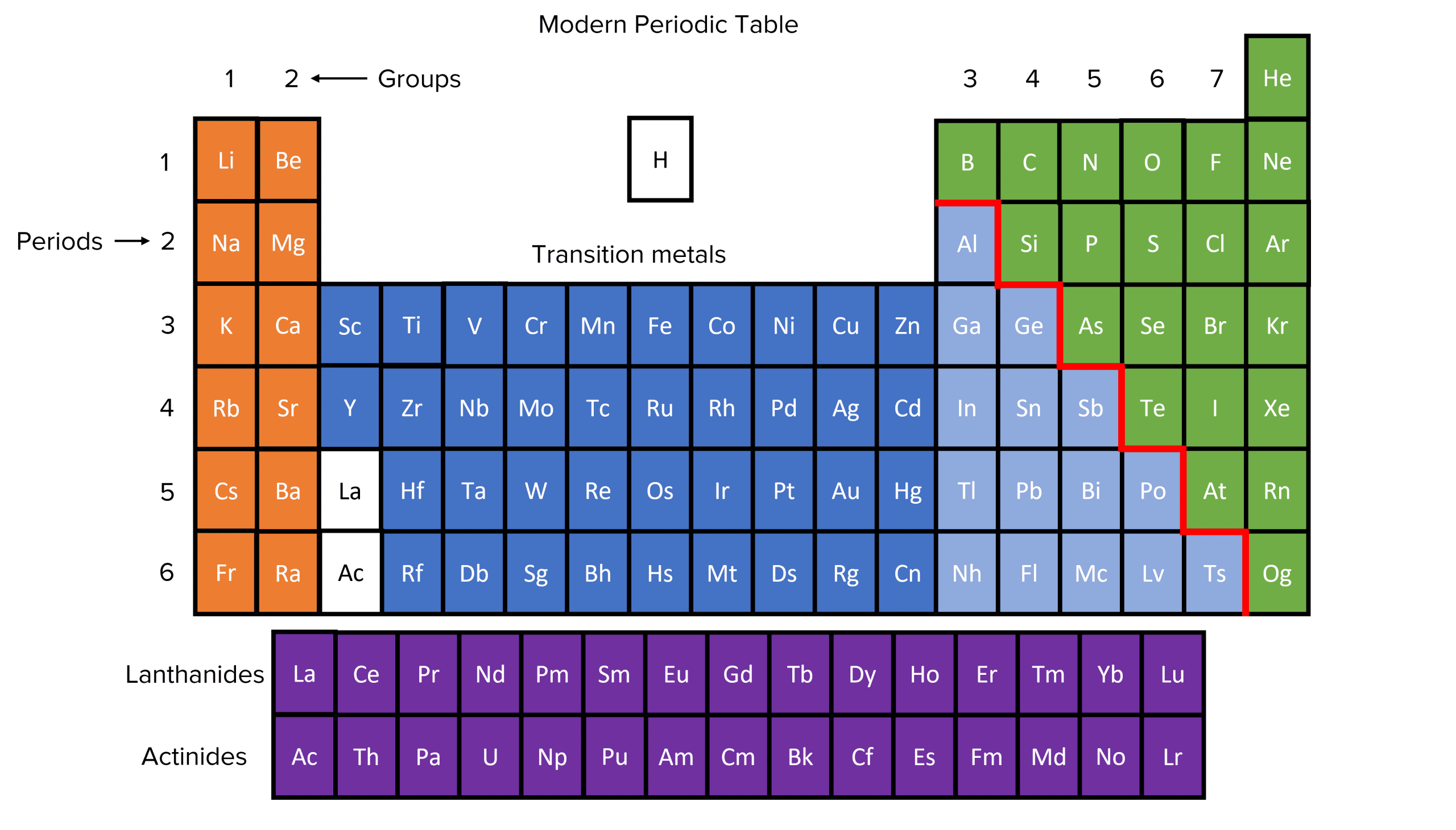
The modern periodic table lists over 100 elements. Now that atomic structure is understood, the modern periodic table organises elements by increasing atomic/proton number. Doing so gives a predictable, periodically repeating pattern of chemical properties that repeats every 8 elements. The 8 columns of the periodic table are called groups. Each group collects elements with similar chemical properties. They have similar chemical properties because each element in a particular group has the same number of electrons in its outer shell, and therefore reacts in similar ways in chemical reactions. The rows of the periodic table are called “periods”. Each period collects elements with the same number of electron shells.
The Periodic Table Example Questions
Question 1: How did early periodic tables order the known elements?
[1 mark]
In order of increasing atomic weight.
Question 2: How does the modern periodic table order the known elements?
[2 marks]
In order of increasing atomic/proton number.
Question 3: State two innovations that Mendeleev made to the periodic table.
[2 marks]
He left gaps and made predictions of the properties of the missing elements.
He ordered the known elements by atomic weight, but changed the order to better fit the chemical properties.
Question 4: Which two elements are from the same group? Why?
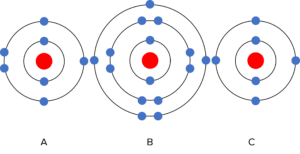
[2 marks]
A and B.
They have the same number of electrons in their outer shell.
Question 5: Which two elements are from the same period? Why?

[2 marks]
A and C
They have the same number of electron shells.





