Giant Structures
Giant Structures Revision
Giant Structures
Many covalent compounds exist as simple molecules, made up of small numbers of atoms (for example water, with only 3 atoms). Some compounds however have much more complex structures. Examples of these structures are polymers, giant covalent compounds, and some unique structures of carbon. The compounds that fit into these categories all contain very large numbers of atoms. In some cases, such as the Buckminster fullerene (see below), this number of atoms is large, but finite (it has a maximum value). In other cases, such as silicon dioxide, this number is theoretically infinite, with the only limit being the number of available atoms.
Polymers
Polymers are a type of covalent molecules , made up of long chains of carbon atoms, held together by covalent bonds. Polymers often contain tens of thousands of atoms, arranged in repeating units. These units are themselves simple covalent molecules. In a single polymer chain there are often thousands of repeating units.
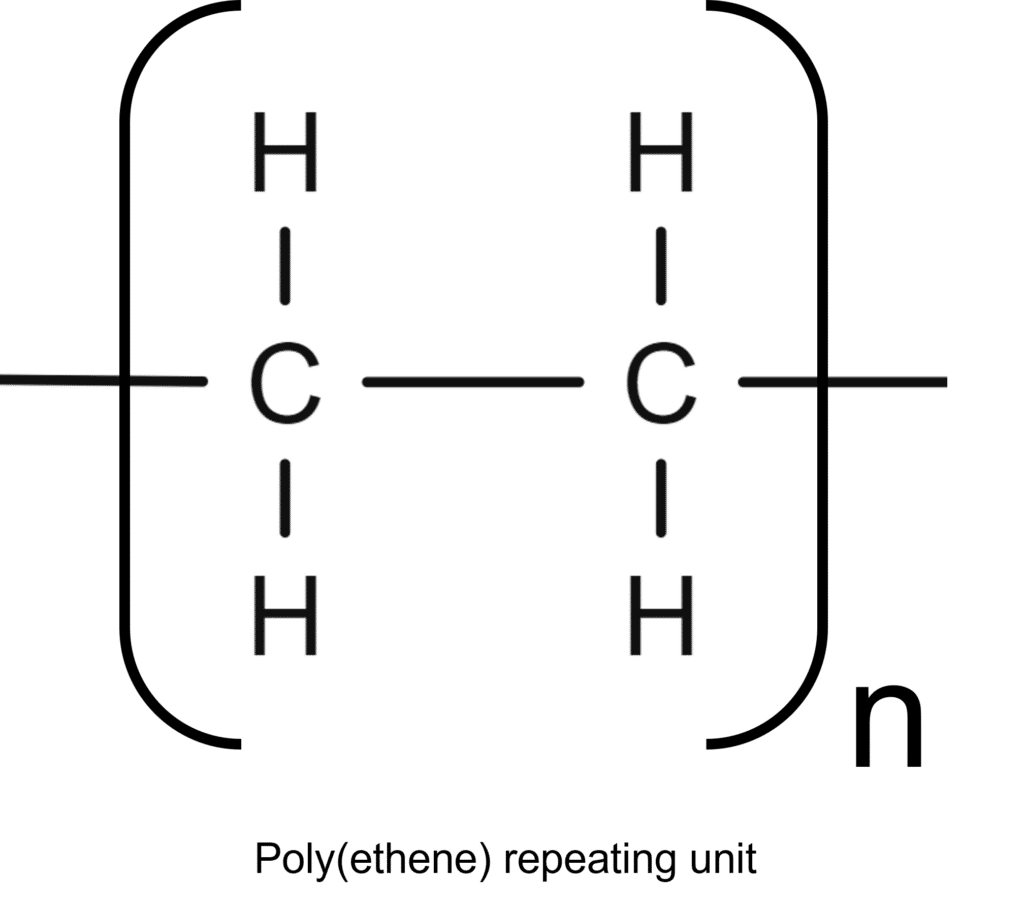
Due to the incredible number of atoms involved, it would be impractical (if not impossible) to draw out the whole structure of a polymer. Instead, the structure of the repeating unit is identified and used as a stand in. To differentiate the repeating unit molecules off the polymer chain from an individual molecule, we wrap the structure in brackets. Next to this, we write the number, n, of repeating units in the chain. When drawing this repeating unit, the bonds at the sides of the molecule are extended outside the brackets to show that connection to the adjacent repeat units.
The formula of a polymer is worked out in a similar way, with the formula of the repeat unit enclosed in brackets, with the number of units, n, in the chain written outside, e.g. \left(\text{C}_2\text{H}_4\right)_\text{n}.
Because polymers are very large, the intermolecular forces between them are much stronger than in simple covalent molecules. This means that most polymers are solids at room temperature. However, these intermolecular forces are still weaker than the electrostatic attractions found in ionic solids, so the melting points of polymers remain much lower.
Giant Covalent Structures
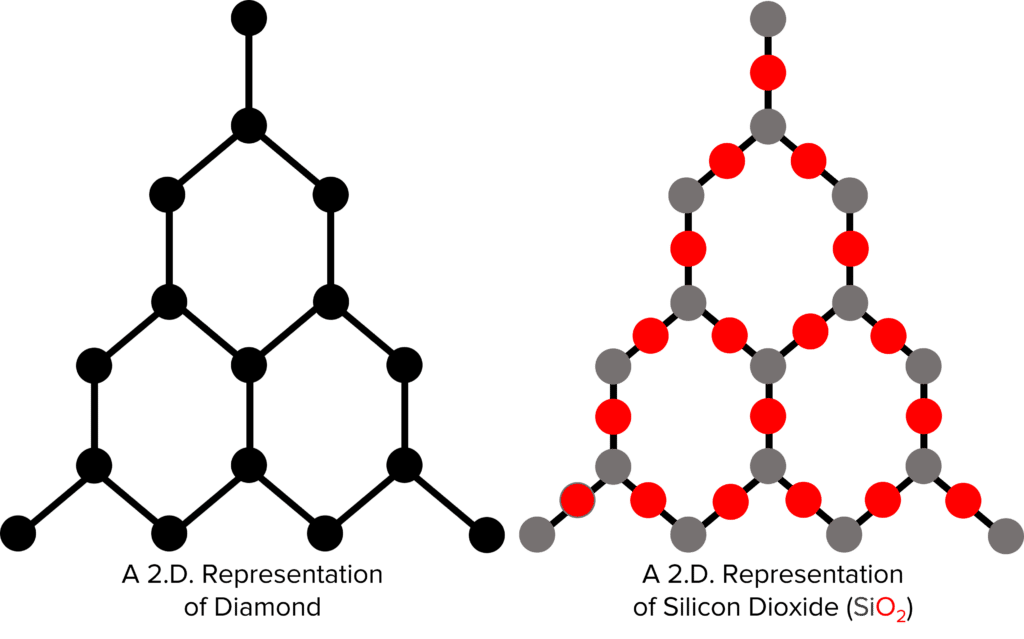
Unlike polymers, there are no distinct molecules in giant covalent structures. Instead, all the atoms in the structure are covalently bonded together into a large lattice. These structures contain billions upon billions of atoms, meaning there are billions upon billions of covalent bonds present as well. This makes giant covalent structures incredibly strong and hard. The most well known example of a giant covalent structure is diamond.
Diamond is the hardest material known to man. This hardness is a result of the billions of strong covalent bonds in its giant covalent structure. These bonds require a lot of energy to overcome and so a lot of force is needed to damage a diamond. Diamond, like all giant covalent structures also has a very high melting point (over 3,900\degree \text{C}). This again is a result of the billions of strong covalent bonds. A serious amount of energy is needed to overcome these bonds.
Other common examples of giant covalent structures are silicon dioxide and graphite (discussed in more detail below).
Structures of Carbon
Carbon is an interesting case when it comes to covalent bonding. Carbon compounds can exist in a range of forms, from simple molecules like CO2 to giant covalent structures such as diamond (see above). Carbon also exits in a few unique structures, with very interesting properties.
Graphite
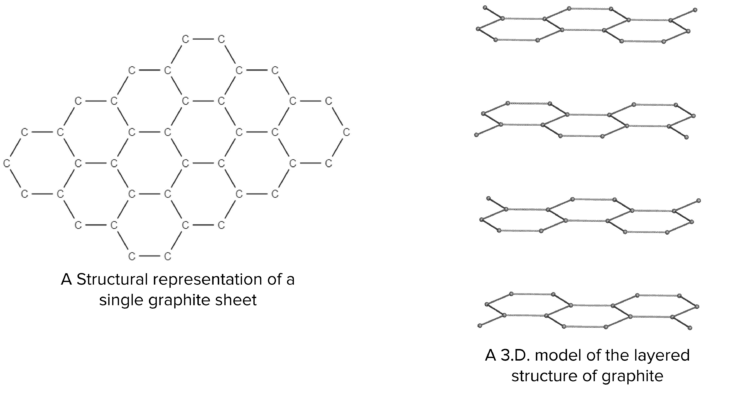
Graphite is a special type of giant covalent structure. In graphite, each carbon atom is bonded to 3 other atoms. Because carbon is able to make 4 bonds, this arrangement leaves each carbon atom with a free electron (delocalised). As a result, graphite is able to conduct electricity thanks to the presence of these delocalised electrons. The carbon atoms in graphite are arranged in flat sheets of connected hexagons.
Each sheet is held together by the strong covalent bonds between the carbon atoms and as such any individual sheet is difficult to break, giving graphite a high melting point. These sheets are then layered on top of one another, held together by weak intermolecular forces. The weakness of these forces mean that the layers of carbon sheets in graphite can slide easily over one another, making graphite very slippery. This is why graphite makes good pencil lead; the layers of graphite can slide off the tip of the pencil and onto the page.
Graphene
Graphene is a singular layer of graphite. Graphene is a very light material (it is only one atom thick) and yet, due to the many strong covalent bongs that hold it together, it is a very strong material. Graphene has a very high melting point (4510\degree \text{C}). It can also conduct electricity thanks to its free electrons. Graphene is a very useful material, used in composites to make other materials stronger with out making them much heavier, and in electronics.
Fullerenes
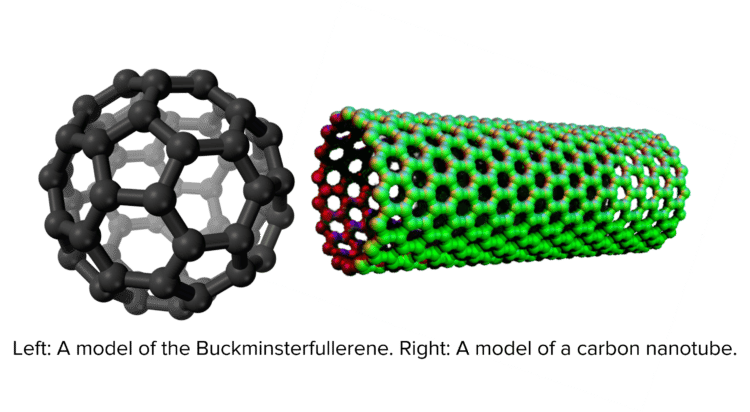
Fullerenes are special types of carbon molecules shaped like either tubes or hollow balls. The first fullerene to be discovered was the Buckminsterfullerene \left(\text{C}_{60}\right), a hollow ball made up of 60 carbon atoms. Carbon nanotubes are long hollow cylinders made up of carbon atoms. The properties of nanotubes make them useful for a wide range of electronic applications. Similar to graphene, carbon nanotubes can be used to strengthen materials without adding much to their weight. Because fullerenes are hollow, they are often used to deliver drugs inside the body or as catalysts for chemical reactions.
Giant Structures Example Questions
Question 1: Explain the properties of graphene in terms of its bonding.
[4 marks]
- Graphene is a single sheet of graphite.
- As such, it has the same bonding between carbon atoms.
- This means that there are many strong covalent bonds, giving graphene a high melting point.
- The bonding in graphene also gives each carbon atom a delocalised electron. As such, graphene can conduct electricity.
Question 2: Explain the melting point of diamond in terms of its structure and bonding.
[3 marks]
- Diamond exists as a giant covalent molecule.
- As such, it has many strong covalent bonds.
- These bonds require a lot of energy to overcome. This gives diamond a high melting point.
Question 3: What does the n represent in a polymer formula?
[1 mark]
The number of repeat units (in the chain).