Hydrocarbons and Alkanes
Hydrocarbons and Alkanes Revision
Hydrocarbons and Alkanes
Hydrocarbons are an incredibly common type of chemical compound. They are characterised by their constituent elements, carbon and hydrogen, and come in a variety of different forms. Hydrocarbons are found throughout the world, from natural fibers to synthetic plastics. The most simple form of hydrocarbon is the alkane.
Hydrocarbons
A hydrocarbon is a molecule that is made up of hydrogen and carbon only.
- They consist of chains of carbon atoms bonded together, with hydrogen atoms bonded to each carbon atom.
- The structures of hydrocarbons are governed by bonding of the carbon atoms. They are able to form up to four bonds with other atoms.
- In practice, this can be four single bonds, one double bond and two single bonds, or two double bonds. A carbon atom may not have more than four bonds at any one time.
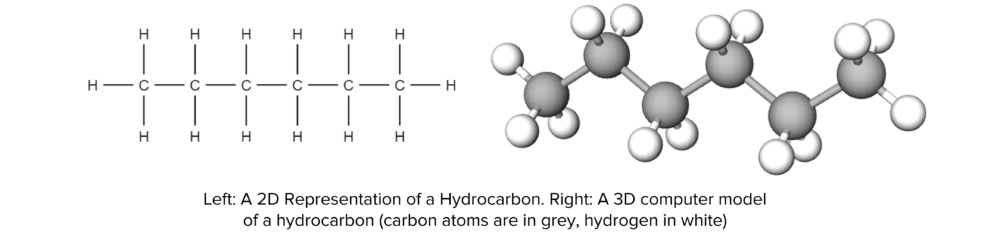
Hydrocarbons come in a wide range of forms, of varying complexity. Hydrocarbon compounds can be divided up into different homologous series. A homologous series (also known as a family) is a group of molecules that have similar properties and all share a general formula. The most simple homologous series are the alkanes.
Alkanes
Alkanes form the most basic series, containing no functional groups, bonded together with only single bonds. Alkanes tend to exist as chains of carbon atoms that can range in sizes from one carbon atom to thousands of carbon atoms long. Each alkane chain has a name based on its length. The first four members of the alkane family are shown below:
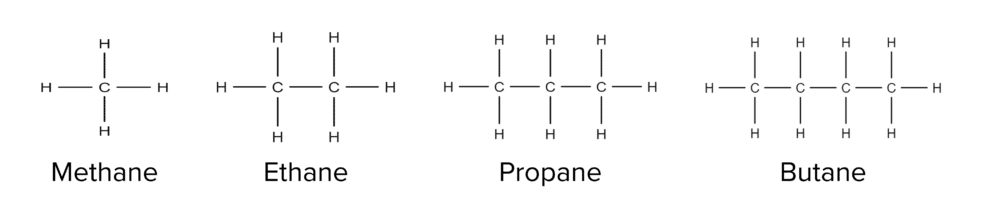
In chemistry, the naming of molecules is strictly governed by as set of rules and these rules are collectively known as nomenclature. The nomenclature for alkanes dictates that each molecules name are made up of a prefix relating to the number of carbons present (e.g pent- for 5 carbon atoms or hex- for 6), and the suffix -ane.
The only exceptions to these rules are the first four alkanes (shown above). These molecules do not have numerical prefixes and instead have unique ones, these are:
- Meth– for 1 carbon.
- Eth- for 2 carbons.
- Prop- for 3 carbons.
- But- for 4 carbons.
Molecular Formulae: Alkanes
All alkanes have a molecular formula that will fit the following general pattern (note than n stands for the number of carbons in the chain):
\text{Alkane General Formula}=\text{C}_n\text{H}_{2n+2}
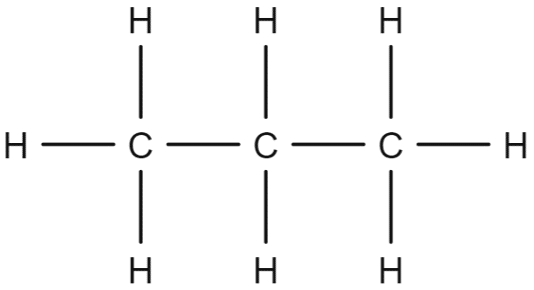
The structure of propane contains three carbon atoms bonded together in a chain, with eight hydrogens attached to said chain. This fits with the general formula given above:
\text{C}_3\text{H}_{\left(2\times3\right)+2}
\text{C}_3\text{H}_{6+2}
\text{C}_3\text{H}_8
We can deduce the general formula by looking at the way in which the hydrogen atoms are arranged in the propane chain. Firstly, we consider the carbon atom that is in the middle of the chain. This one carbon atom is bonded to two other carbon atoms either side of it. This leaves it with two free bonds that can be made to hydrogen atoms.
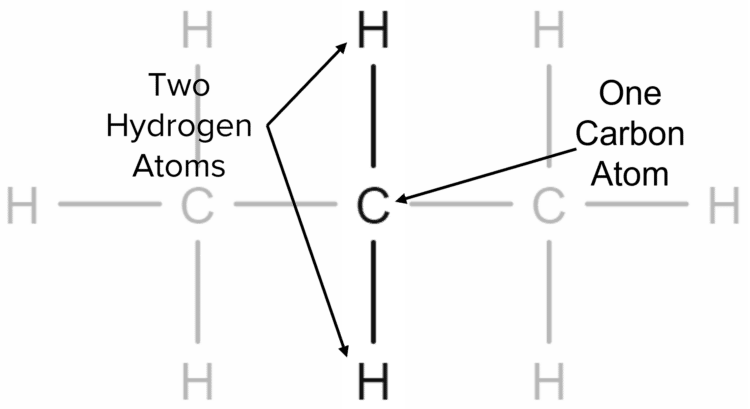
We can say therefore that, for every one carbon atom in the centre of the propane chain, there are two hydrogen atoms. This can be made more general by saying that for every carbon atom in centre of an alkane chain, there are two hydrogen atoms. Therefore, in the centre of an alkane chain we have double the number of hydrogen atoms as there are carbon atoms.
Next, we consider the end of the chain. Here we have one carbon atom bonded to one other carbon atom on one side, leaving three free bonds for hydrogen atoms.
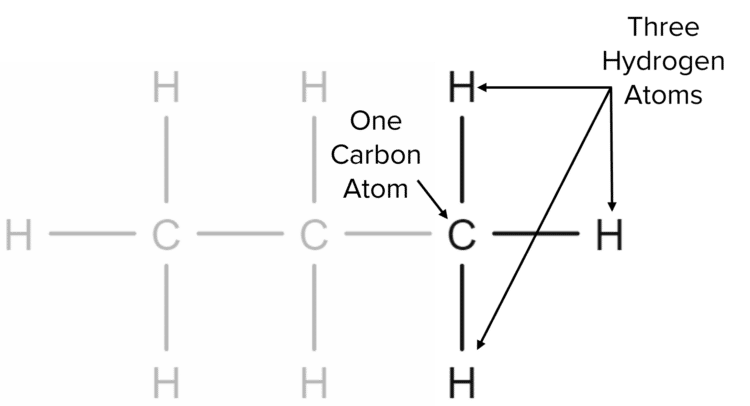
Here we have an additional hydrogen atom bonded to the carbon at the end of the chain. As the chain has two ends, there must be two additional hydrogens in the structure, one at each end. We can again generalise this to say that, for each end carbon in an alkane chain, there is one additional hydrogen atom.
Putting this together with the central carbon atoms, this gives us a general formula of:
\text{C}_n\text{H}_{2n+2}
Properties of Alkanes
Alkanes show a number of common properties, such as their inability to mix with water, having relatively low boiling points, and flammability. These properties are all influenced by the length of the alkane chain in question. Three important trends are those in the melting and boiling points of alkanes, the flammability alkanes, and their viscosity (i.e. how runny or slippery a liquid is).
Melting and Boiling Points
The melting and boiling points will both increase as the length of the alkane chain increases. As the chains get longer, the intermolecular forces between them increase in strength. As such, more energy is required to overcome these forces, leading to a higher temperature required to melt or boil the alkane.
Flammability
As the length of alkane chains increases, their flammability decreases.
Viscosity
The viscosity of an alkane also increases as chain length increases. This is because the attraction between alkane chains increases as the chain length increases. This makes it more difficult for the chains to move over one another and spread out, creating thicker and less mobile liquids.
The Combustion of Alkanes
The term combustion means to burn in oxygen. When alkanes undergo combustion, they are oxidised by oxygen to form water and carbon dioxide. This process is highly exothermic and releases a lot of energy and so hydrocarbons are very commonly burned to produce heat (e.g. a propane fuelled camping stove) or as fuels in electricity production (e.g. in oil fired power stations). This release in energy comes from the large number of hydrogen-oxygen and carbon-oxygen bonds that are formed during combustion. As the length of an alkane increases, the energy released upon its combustion increases as well, as there are more carbon and hydrogen atoms available to form bonds with oxygen. The combustion of alkanes can either be complete or incomplete.
Complete Combustion
In complete combustion, there is an excess of oxygen and so all of the atoms of the alkane are oxidised to form carbon dioxide and water:
\text{Alkane}+\text{Excess Oxygen}\rarr\text{Carbon Dioxide}+\text{Water}
If the molecular formula of an alkane is known, it is possible to construct a balanced symbol equation for its combustion. Take the example of the complete combustion of propane:
\text{C}_3\text{H}_8+\text{O}_2\rarr\text{CO}_2+\text{H}_2\text{O}
First, we must balance the carbon atoms. On the left hand side of the equation there are three carbon atoms in the propane molecule. On the right there is only one. To balance this we need to have three \text{CO}_2 molecules:
\text{C}_3\text{H}_8+\text{O}_2\rarr 3\text{CO}_2+\text{H}_2\text{O}
Secondly, we need to balance the hydrogen atoms. On the left hand side there are eight, on the right there are only two. To balance this we need to have four water molecules:
\text{C}_3\text{H}_8+\text{O}_2\rarr 3\text{CO}_2+4\text{H}_2\text{O}
Finally, we must balance the oxygen atoms. On the left we have two, on the right we have ten, six from the carbon dioxide and four from the water. As such we need five oxygen molecules on the left:
\text{C}_3\text{H}_8+5\text{O}_2\rarr 3\text{CO}_2+4\text{H}_2\text{O}
Incomplete Combustion
In incomplete combustion, there is not enough oxygen present to oxidise all of the atoms of the hydrocarbon. This will lead to the production of carbon monoxide \left(\text{CO}\right) and or soot instead or carbon dioxide. Incomplete combustion will burn with a yellow and smoky flame.
Hydrocarbons and Alkanes Example Questions
Question 1: Define the term ‘Hydrocarbon’.
[1 mark]
A compound that consists of hydrogen and carbon only.
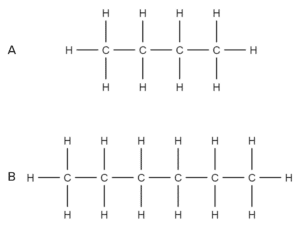
Question 2: State which of the following compounds you would expect to boil at a lowest temperature:
Explain your answer.
[4 marks]
- Compound A will boil at the lowest temperature.
- Compound A is a shorter alkane than compound B.
- As such the intermolecular forces between molecules of A are weaker than those between molecules of B.
- This means that less energy is required to overcome them.
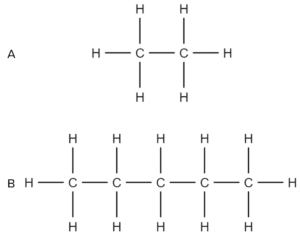
Question 3: State which of the following compounds you would expect to release the most energy when burned:
Explain your answer.
[4 marks]
- Compound B will release the most energy when burned.
- Compound B is the longer alkane chain.
- The energy released when burning alkanes increases as chain length increases.
- So as it is the longest chain, B will release the most energy.
Question 4: From its molecular formula, deduce weather the following compound is an alkane:
\text{C}_6\text{H}_{14}
[4 marks]
- The compound is an alkane.
- The compounds molecular formula fits the general formula of an alkane.
- There are 6 carbon atoms so 2n+2 should equal 14.
- There are 14 hydrogen atoms.
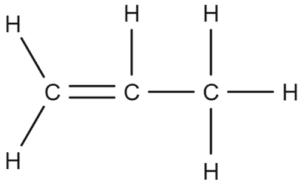
Question 5: State whether or not the following compound is an alkane:
Explain you answer.
[3 marks]
- The compound is not an alkane.
- The compound contains a double bond.
- Alkanes contain only single bonds.
(Allow one mark for presentation of alkane general formula.)





