Crude Oil
Crude Oil Revision
Crude Oil
Crude oil is a finite natural resource and is used for a variety of purposes. To use crude oil, it must first be refined. This is done using a technique called fractional distillation. The compounds in crude oil can be broken up into smaller chains using a separate technique called cracking.
Fossil Fuels and Crude Oil
Fossil fuels are substances that are produced from the remains of living organisms that existed millions of years ago. The most famous (or perhaps infamous) of these substances is crude oil.
Crude oil is formed when living organisms die and become buried under tons of rock and sediment. These organisms often lived in the Earth’s oceans, falling to the sea bed when they died. Over time, the remains of these organisms became buried under many layers of sediment, the weight of which created a huge amount of pressure. Over millions of years, this pressure caused the organic compounds that made up these organisms to liquify.
Today, this liquid is extracted from deep within the Earth’s crust as crude oil.
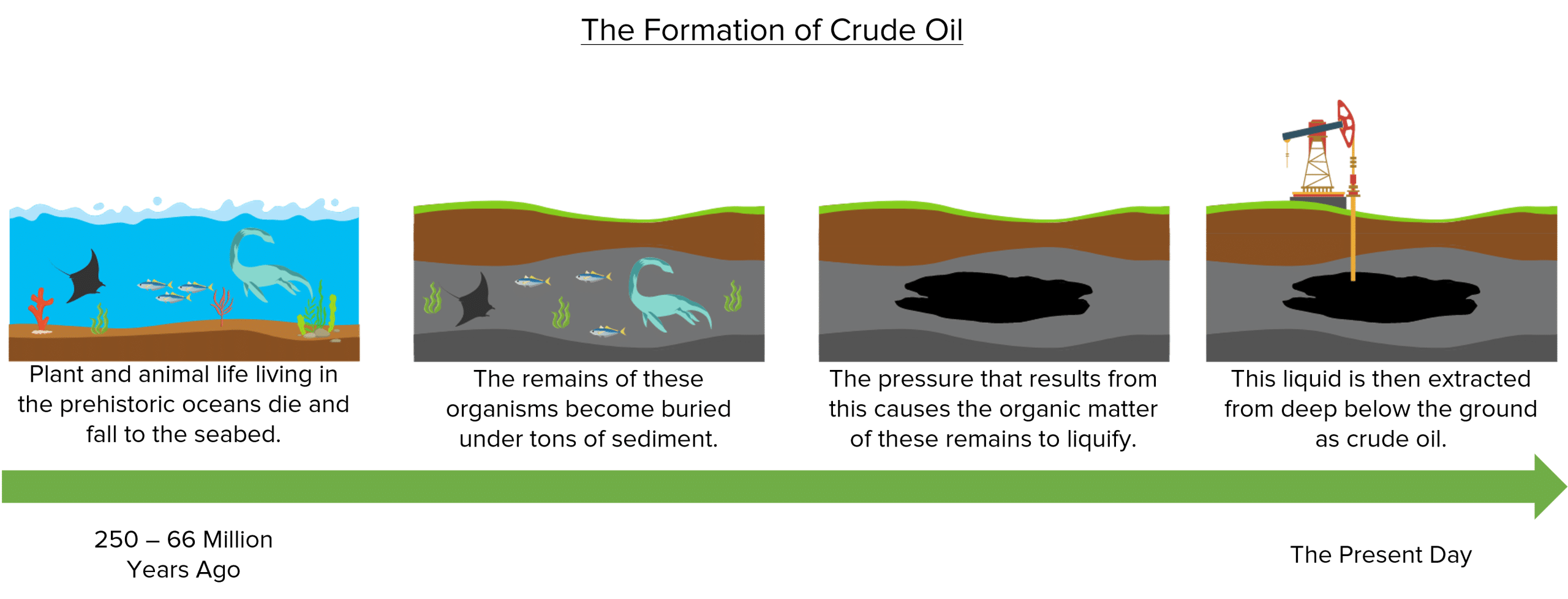
Because crude oil takes millions of years to form, it is known as a non-renewable resource. This means that we use crude oil at a much faster rate than it can be replaced. As a result, once we have used up the crude oil that currently exists, it is gone forever.
Crude oil itself is a mixture of different hydrocarbon compounds of varying sizes. The hydrocarbons that are contained within crude oil have many different uses, from petrol to plastics. For this reason, crude oil is called a feedstock. These hydrocarbons will also have a range of different properties that will depend on their size. These properties include their colour, viscosity, and melting and boiling points.
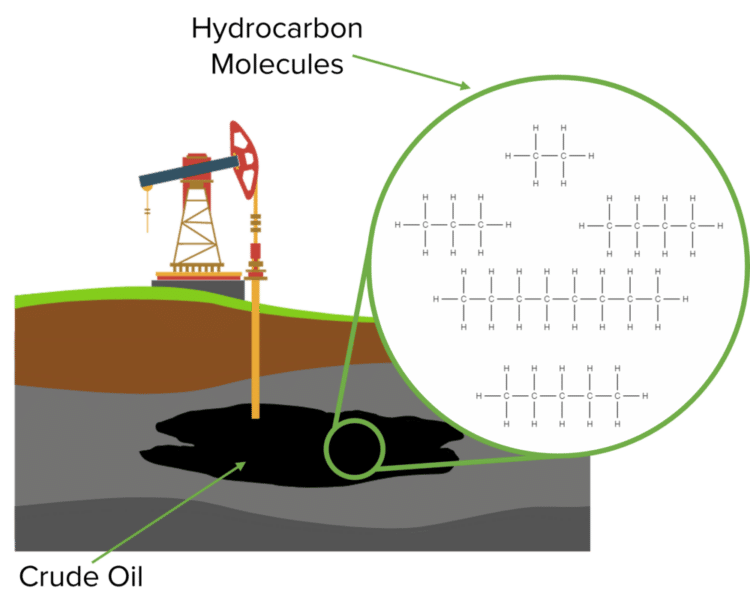
Uses of Crude Oil
Crude oil is fairly useless on its own. Instead crude oil is useful as a feedstock for the production of other compounds used for a range of different purposes. The compounds that can be broadly split into two categories, fuels and petrochemicals.
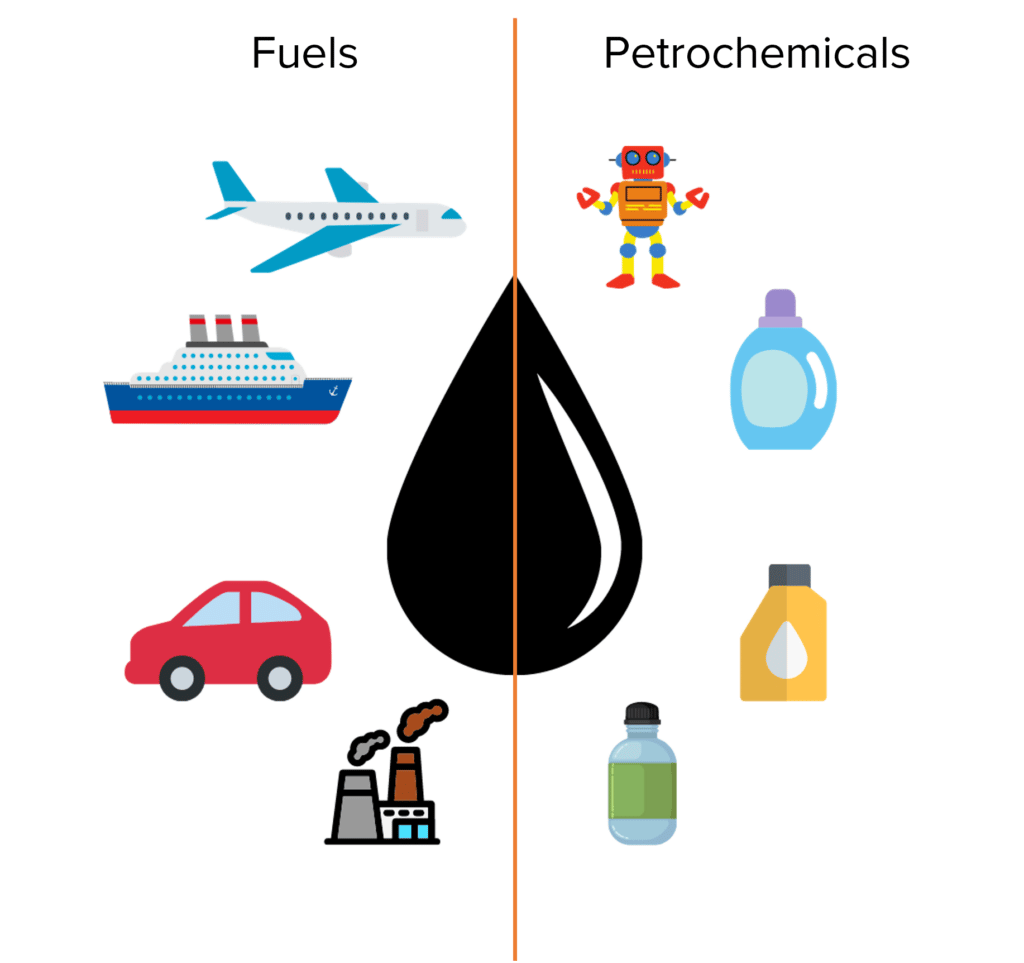
Crude oil contains many of the fuels that power everyday life across the world. Kerosene (used as jet fuel), petrol and diesel (used to power cars) and heavy fuel oil (used in shipping and in power stations) all come from crude oil.
Crude oil also provides a source of starting materials for the production of many every day chemicals. Chemicals produced from the materials found within crude oil are known as petrochemicals and include solvents, lubricants, detergents, and polymers.
For any of these compounds to be useful however, they must first be extracted from the mixture of crude oil. To do this an industrial technique know as fractional distillation is used.
Fractional Distillation
In fractional distillation, the compounds contained within crude oil are separated out into groups called fractions, according to their boiling points. Fractions are groups of molecules that are made up of similar numbers of atoms. These compounds will have similar boiling points, allowing them to be separated from other fractions.
Fractional distillation follows the following process:
- Crude oil passed through a furnace where it is heated up to around 350\degree \text{C}. This causes the hydrocarbon compounds in the crude to evaporate.
- The resulting mixture of gaseous hydrocarbons is then fed into a distillation column and allowed to rise up. The column is kept very hot at the bottom (still around 350\degree \text{C}) but allowed to cool along its height.
- As the gasses rise in the column they begin to cool and condense . How high in the column a given gas will rise before condensing will depend on its boiling point. Compounds with similar boiling points will evaporate at similar heights, forming fractions.
- The condensed fractions are then collected and sent out for use as fuels or in the petrochemicals industry.
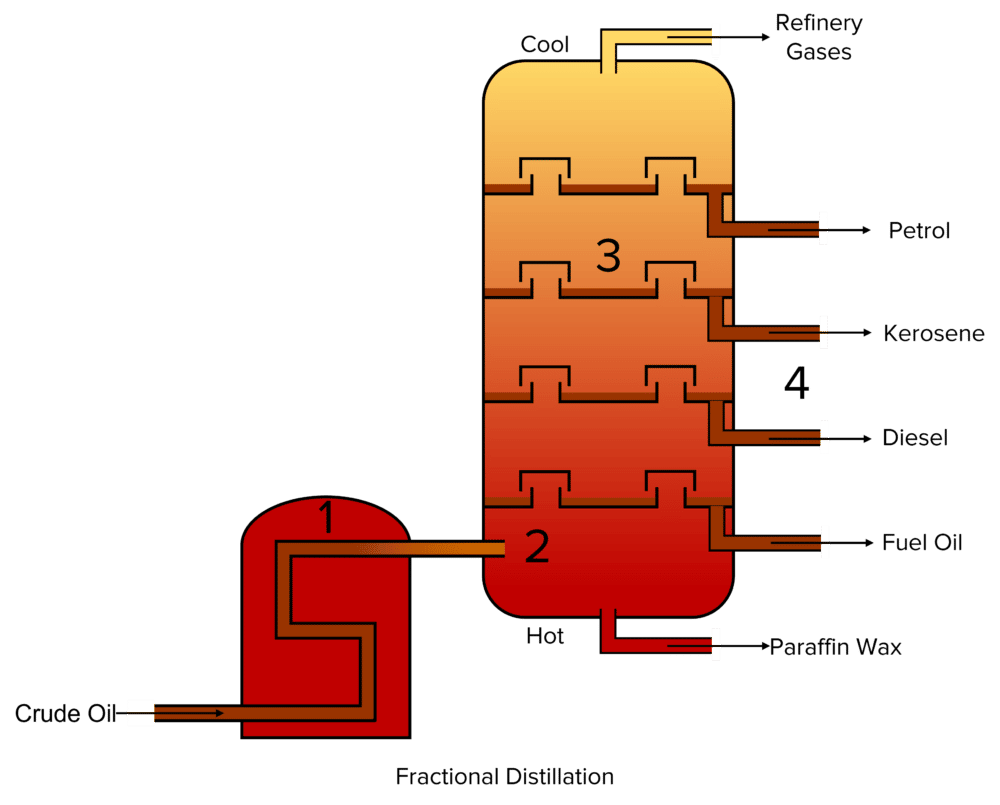
Fractional distillation relies on the relationship between a compounds size and it’s physical properties to separate hydrocarbons out into fractions.
- Fractions containing the smallest hydrocarbons will also have the lowest boiling points. This means that they also condense at the lowest temperatures. Therefore, these compounds rise all the way through to the top the distillation column before they are condensed and collected. The hydrocarbons with the shortest chains are collected at the top of the column. This includes compounds such as petrol. Additionally, some compounds are so small they do not condense at all. These are collected as refinery gasses.
- Fractions containing medium sized hydrocarbons will also have medium boiling points. This means that they also condense at medium temperatures. Therefore, these compounds only rise about halfway up in the distillation column before they are condensed and collected. The hydrocarbons with medium length chains are collected from the middle of the column. This includes compounds such as kerosene and diesel.
- Fractions containing the largest hydrocarbons will also have the highest boiling points. This means that they also condense at the highest temperatures. Therefore, these compounds do not rise up very high in the distillation column before they are condensed and collected. The hydrocarbons with the longest chains are collected at the bottom of the column. This includes compounds such as paraffin wax and fuel oil.
Once distilled, the individual hydrocarbons obtained can be further broken down using a process called cracking.
Cracking
Cracking, unlike fractional distillation, is a chemical process. Where as fractional distillation takes advantages of the different physical properties of different molecules to separate them from one another, cracking uses thermal decomposition to break apart individual molecules. This is done to produce more useful and shorter chain alkanes from longer and less useful ones. As the flammability of hydrocarbons decreases as their length increases, it is desirable to have shorter hydrocarbons for use as fuels.
When cracked, a long chain alkane will produce one shorter chain alkane as well as an alkene.
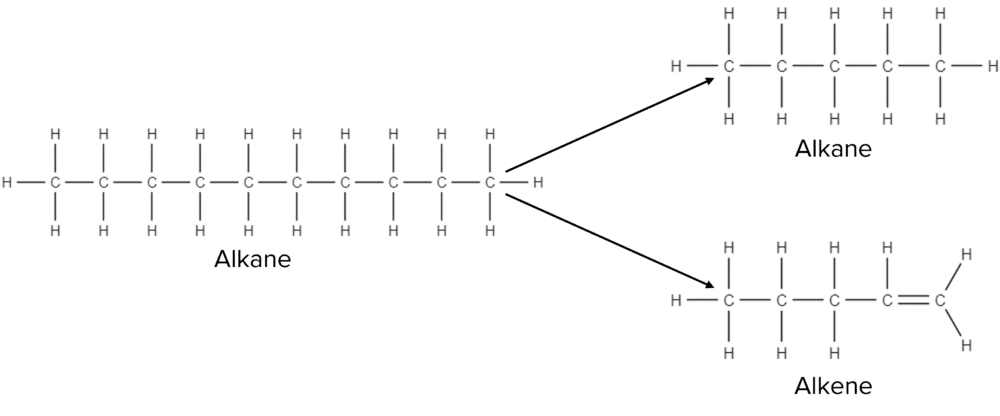
There are two ways to crack an alkane; the first is to use catalytic cracking, the second is to use steam cracking. Both methods require high temperatures and a fairly high pressure.
Catalytic Cracking
In catalytic cracking, long chain hydrocarbons are first heated up and vapourised. This vapour is then passed over a hot powdered aluminum oxide catalyst. The long chain molecules will break apart on the surface of the catalyst, producing shorter chain alkanes and alkenes.
Steam Cracking
In steam cracking, the long chain alkane is again vapourised. It is then mixed with steam and then heated to a higher temperature. The long chains will then break down into shorter alkanes and alkenes.
When constructing balanced equations for cracking reactions it is important to remember that the total number of carbon and hydrogen atoms shared across the alkane and alkene should always equal the total number of carbon and hydrogen atoms in the original alkane. For example, the balanced equation for the reaction above would be:
\text{C}_{10}\text{H}_{22}\rarr\text{C}_5\text{H}_{12}+\text{C}_5\text{H}_{10}
Crude Oil Example Questions
Question 1: Bitumen is a long chain hydrocarbon in road construction.
Predict where you would collect bitumen from a fractional distillation column.
Explain your answer.
[3 marks]
Prediction: At the bottom of the column.
Explanation: Bitumen is a long chain hydrocarbon. As such it has a high boiling point. This means it is extracted at the bottom of the column where it is hottest.
Question 2: Petrol is a hydrocarbon fuel that can be extracted from crude oil. Name two other products than can be produced from crude oil.
[2 marks]
Any two from:
- Kerosene
- Paraffin Wax
- Bitumen
- (Heavy) Fuel Oil
- Detergent
- Lubricant
- Solvents
- Polymers
Question 3: The alkane dodecane \left(\text{C}_{12}\text{H}_{26}\right) is cracked to form the ethene \left(\text{C}_2\text{H}_4\right) and an alkane. Give the structural formula of this alkane.
[2 marks]
\text{C}_{12-2}\text{H}_{26-4}
\text{C}_{10}\text{H}_{22}






