Alkenes
Alkenes Revision
Alkenes
Alkenes are another family of the hydrocarbon group of molecules. The presence of alkenes can be revealed using bromine water and they will burn with a smoky flame. They undergo a number of addition reactions including; hydrogenation, reaction with steam, and reactions with halogens.
What is an Alkene?
Alkenes are a homologous series of hydrocarbon molecules. They are very similar to alkanes in that they contain carbon and hydrogen atoms only. Unlike alkanes, that contain only single bonds, alkenes contain one or more double bonds between carbon atoms. These double bonds are known as functional groups. A functional group is a group of atoms within an hydrocarbon that determine the chemical properties and reactions of the molecule.
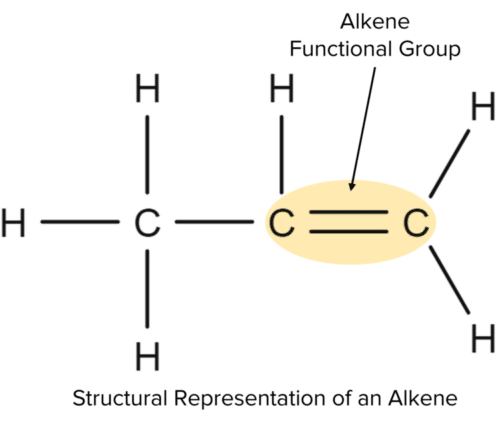
Because alkenes contain double bonds they are referred to as unsaturated. This means they can contain carbon atoms with double bonds, allowing more atoms to be added to them. This is in contrast to alkanes which contain only single bonds, making them saturated.
Nomenclature: Alkenes
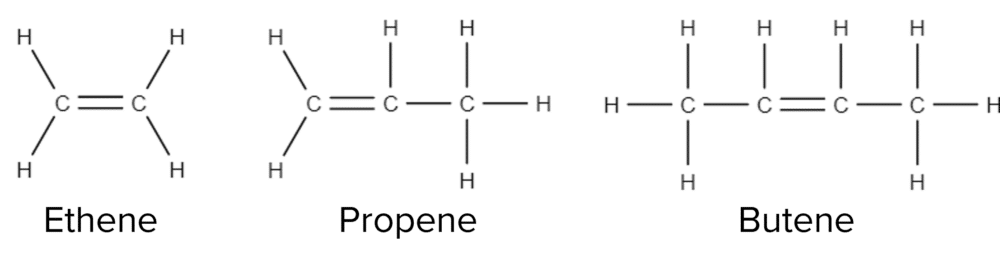
Alkenes have a similar nomenclature alkenes. The first alkenes in the series all have generic prefixes; eth-, prop-, and but-, with the rest having numerical prefixes (e.g. pent-, hex-, hep-). The suffix for alkenes is also similar to that of alkanes. To denote an alkene we use the suffix -ene.
In the case of the alkenes, it is the first three molecules of the family that have generic names. As an alkene has to contain a double bond between carbon atoms, it is impossible to have an alkene of just one carbon.
Molecular Formulae: Alkenes
All alkenes have a molecular formula that will fit the following general pattern:
\text{Alkene General Formula}=\text{C}_n\text{H}_{2n}
This molecular structure can be illustrated using the example of butene. Butene, with a molecular formula of \text{C}_4\text{H}_8 can be represented structurally as follows:
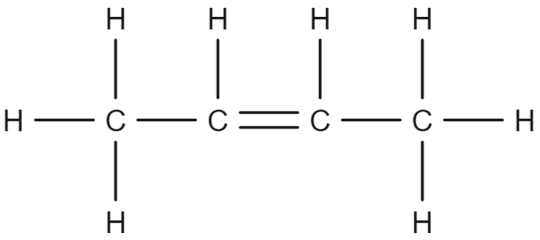
We see that there are four carbon atoms in the chain and eight hydrogen atoms attached. This satisfies the general formula above, with \text{n}=4 and 2\text{n}=8. Unlike alkanes, we do not need to add two additional hydrogen atoms. This is because, for every double bond, we lose two hydrogen atoms.
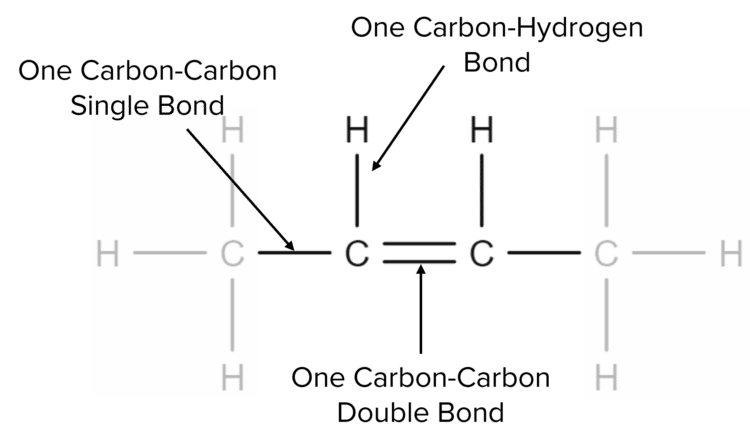
When a double bond is present between two carbon atoms, each atom is only able to make two other single bonds. This means that that the carbon atoms must each lose one bond to hydrogen, leading to two fewer hydrogen atoms in the formula.
Incomplete Combustion
When alkenes are burnt in oxygen, they often undergo incomplete combustion. Incomplete combustion happens when a hydrocarbon compound is burnt without enough oxygen to ensure complete combustion. When alkenes undergo complete combustion they produce water and carbon dioxide.
When alkenes undergo incomplete combustion, a carbon dioxide and water are still produced, but in addition to these, carbon soot and carbon monoxide are also produced. Carbon monoxide is a poisonous gas and can lead to deaths if inhaled in large quantities. The incomplete combustion of an alkene is a much less exothermic process than complete combustion producing a smoky and yellow flame.
It is possible to construct balanced equations for the incomplete combustion of alkenes, for example the incomplete combustion of butene is as follows:
\text{C}_8\text{H}_{10}+5\text{O}_2\rarr2\text{CO}+2\text{CO}_2+4\text{H}_2\text{O}
Alkene Reactions
Alkenes are able to undergo a range of chemical reactions due to their unsaturated nature.
Unsaturated hydrocarbons are able react to as they have the space to accommodate new atoms in their structure. Alkenes undergo a number of reactions that are collectively know as addition reactions. In an addition reaction, the double bond of an alkene is opened up and to form a single carbon-carbon bond and one or more single bonds to other elements:
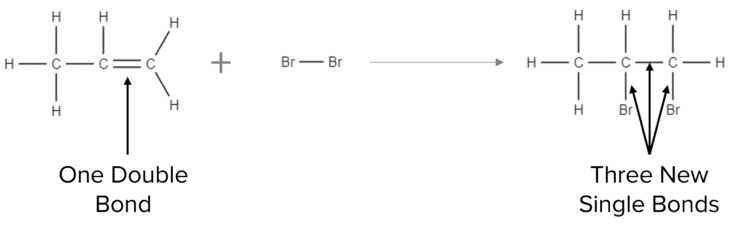
There are generally three types of addition reaction that alkenes can undergo; hydrogenation, steam reactions, and halogen reactions.
Hydrogenation
When hydrogen is added to an alkene the addition is called a hydrogenation reaction. In the presence of a catalyst the \text{H}_2 molecule is able to react with the double bond of the alkene and open it up. This frees up two bonds on each carbon, allowing two new bonds to form between carbon and hydrogen atoms. This results in the formation of a saturated alkane chain.
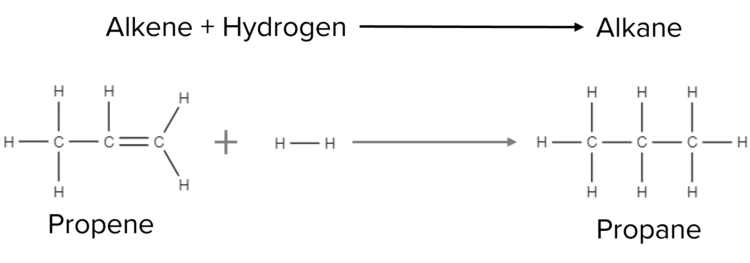
For example, when hydrogen is added to propene, the two react to form propane:
\text{C}_3\text{H}_6+\text{H}_2\rarr\text{C}_3\text{H}_8
Steam Reactions
Alkenes can also react with water in gaseous form. When steam reacts with alkenes, the double bond is opened. The molecule then gains two new hydrogen atoms and a new functional group called an alcohol. This process is also known as hydration:
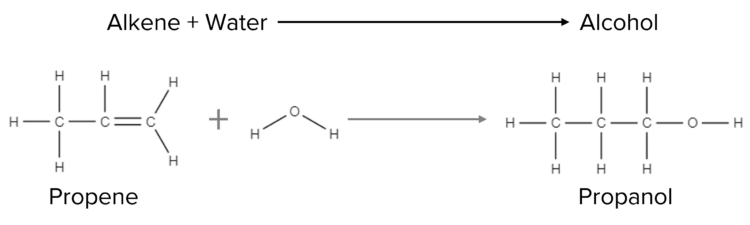
In hydration reactions, a mixture of an alkene and steam are passed over a catalyst. This is the process used in industry to produce ethanol:
\text{C}_2+\text{H}_4\text{H}_2\text{O}\rarr\text{CH}_3\text{CH}_2\text{OH}
Halogen Reactions
Finally, alkenes can react with halogens (group 7 of the periodic table) to form saturated molecules that contain atoms of the halogen. An example of one of these addition reactions is shown above. In this case, propene reacts with bromine to form a new molecule called dibromopropane (shown in first diagram above):
\text{C}_3\text{H}_6\text{Br}_2
This reaction forms the basis of the test used to identify alkenes in solution.
Testing for Alkenes
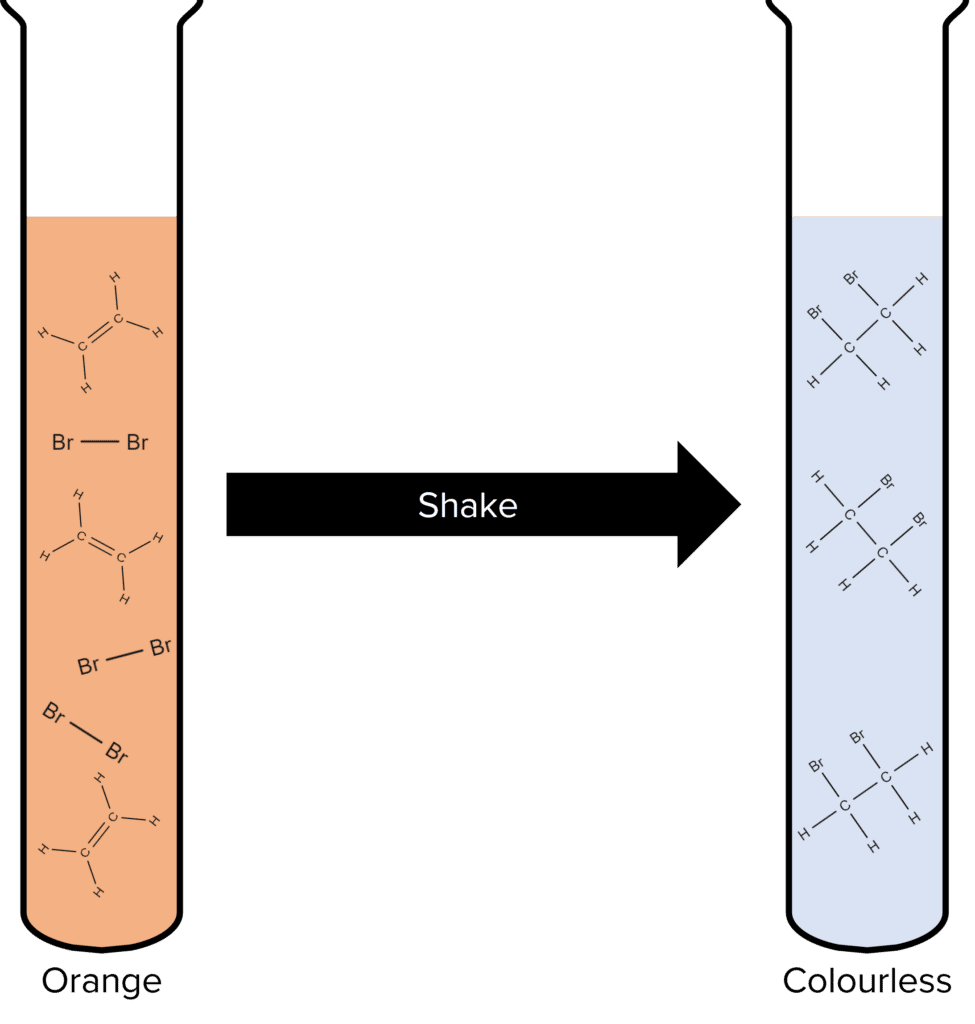
The ability of alkenes to react with halogens means that they can be identified in solutions using chemical tests. Solutions of halogens are often coloured and by observing the change in colour when they react which substances, we can deduce if alkenes are present in said substance.
The most common halogen used to test for alkenes is bromine. Bromine exists as an orange liquid at room temperature and so it can be easily used in chemical tests.
To test for alkenes using bromine, a sample of a solution suspected of containing alkenes should be added to a test tube filled with orange bromine water. The test tube should be shaken and, if the solution turns from orange to colourless, alkenes are present.
This test works because the alkenes are able to undergo addition reactions with the bromine molecules. When the alkene is added to the bromine water it reacts and the bromine molecules break up and bond to the carbon atoms of the alkene. This removes bromine molecules from solution, replacing them with bromoalkenes. As bromoalkenes are colourless, the solution loses its orange colour.
Alkenes Example Questions
Question 1: Which of the following structures does not show an alkene:
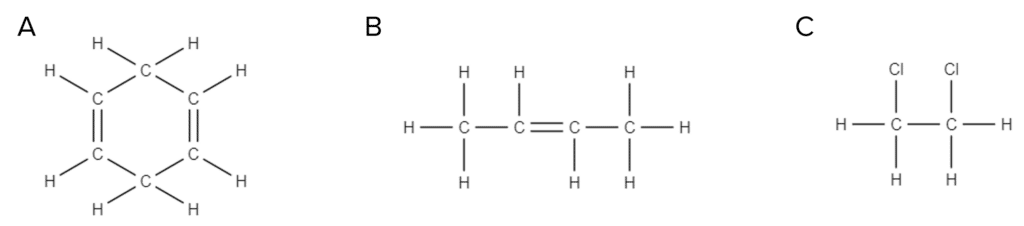
[1 mark]
Question 2: Predict the formula of the resulting compound when butene \left(\text{C}_4\text{H}_8\right) reacts with steam.
[2 marks]
\text{Structure: C}_4\text{H}_9\text{OH}
Question 3: State whether or not the following formula follows the general formula for an alkene:
\text{C}_7\text{H}_{16}
Explain your answer.
[3 marks]
The formula does not follow the general formula of an alkene.
The number of carbons, n, in the formula is 7. If the formula followed the general structure of an alkene then we would expect there to be 14 \left(2n\right) hydrogens.
As there are 16 hydrogen atoms in the formula, it does not follow the formula for alkenes.
(Allow one mark for presentation of general alkene formula.)
Question 4: Describe a test for alkenes.
[3 marks]
- A sample of solution that may contain alkenes is added to a test tube of orange bromine water.
- If the solution changes from orange to colourless, there are alkenes in the solution.