Alcohols
Alcohols Revision
Alcohols
Alcohols are another family of molecules that can be produced from hydrocarbons. Alcohols have a wide range of uses, from disinfectants to consumables. They are able to undergo a range of reactions, including with sodium, water, oxygen, and oxidising agents. Alcohols can be produced from the fermentation of sugars by using yeast.
What is an Alcohol?
Alcohols are a homologous series of molecules. Unlike, the alkanes or alkenes, alcohols do not contain only hydrogen and carbon atoms. Alcohols all contain an alcohol functional group. This group consists of an oxygen and hydrogen bonded together, with a bond between the oxygen atom and the hydrocarbon chain.
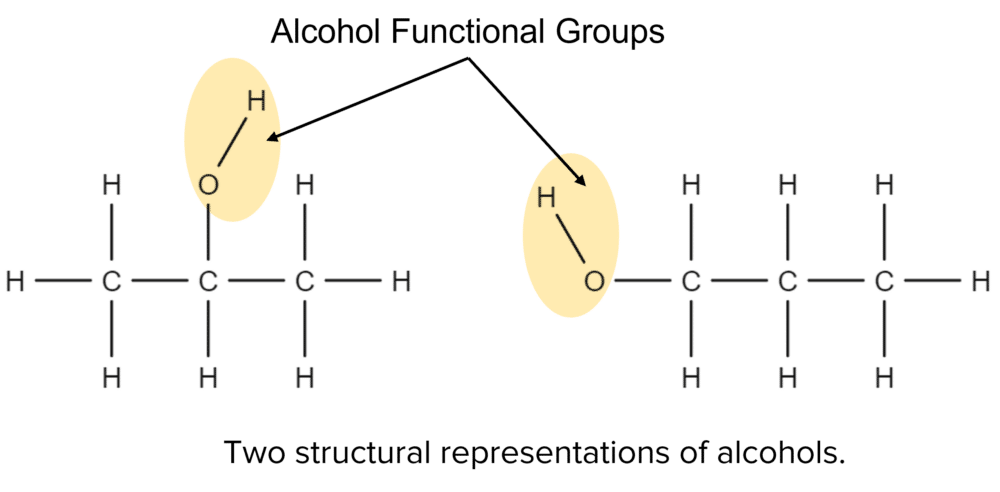
Alcohols follow the general pattern of alkanes and alkenes when it comes to their nomenclature. The first four members of the series have generic prefixes, meth-, eth-, prop-, and but-, with the rest having numerical prefixes. Alcohols are then given the suffix -anol. For example, the structure shown above contains three carbons and an alcohol functional group It’s name will there for be propanol.
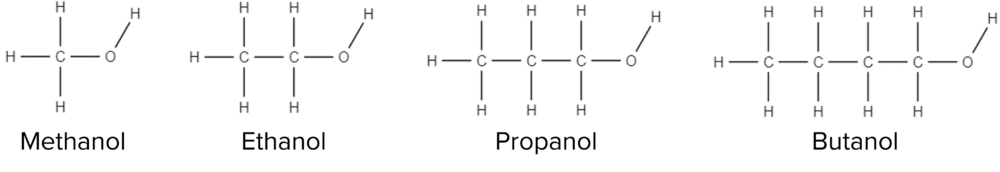
Molecular Formulae: Alcohols
The molecular formulae of alcohols are constructed in a different way to the formulas of alkanes and alkenes. For alcohols, individual carbon atoms are written separately. Take for example the alcohol propanol:
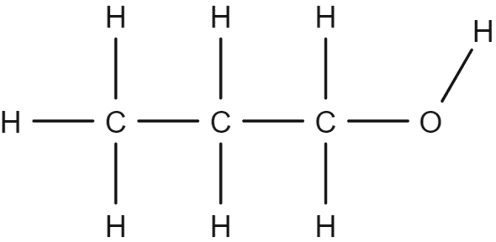
The molecular formula of propanol can be given as \text{CH}_3\text{CH}_2\text{CH}_2\text{OH}. This formula contains three distinct parts, each corresponding to one carbon atom from the alcohol chain. The \text{CH}_3 group will correspond to the end of the chain:
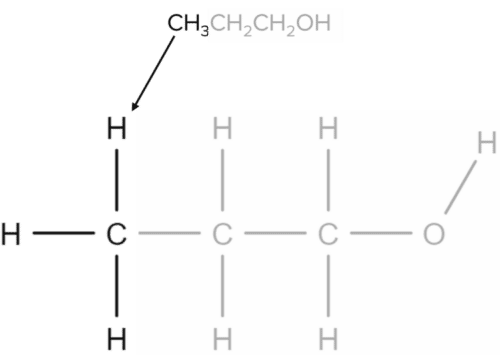
The \text{CH}_2 group represents the middle carbon atom, bonded to two carbons either side and two hydrogens:
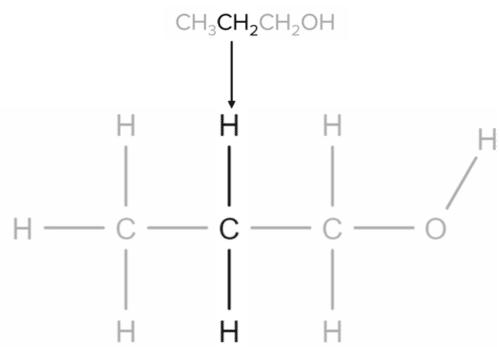
Lastly, the \text{CH}_2\text{OH} group represents the carbon atom to which the alcohol functional group is attached:
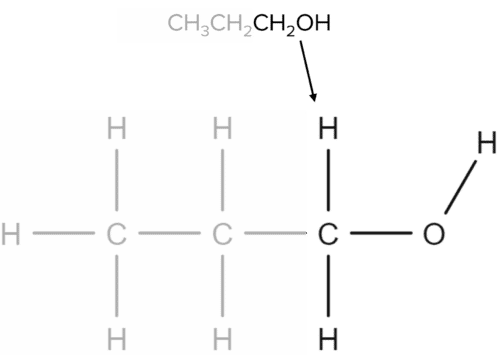
In this case, the alcohol group is located at the end of the chain, giving the \text{CH}_2\text{OH} group in the molecular formula. It is possible for the alcohol to exist in the middle of the chain however and this would give a formula of \text{CH}_3\text{CHOH}\text{CH}_3.
Properties and Reactions of Alcohols
Alcohols all have similar properties. For example, alcohols are flammable in air. When alcohols are burned they will produce carbon dioxide and water:
\text{CH}_3\text{CH}_2\text{OH}+2\text{O}_2\rarr 2\text{CO}_2 + 3\text{H}_2\text{O}
This flammability means that alcohols can be burnt as fuels. Unlike alkenes, alcohols burn with a relatively clean flame, making them much less unpleasant to use as fuels. An example of an alcohol fuel is the use of ethanol in a spirit burner. Alcohols also have a other useful properties:
- Alcohols are also used as solvents.
- Alcohols are able to dissolve most things that will dissolve in water as well as many things that can’t.
- Alcohols are ideal for removing organic chemicals such as fats, oils, and grease.
The first four alcohols are all soluble in water, producing neutral solutions. These solutions are able to react with sodium to form sodium salts and hydrogen gas:
2\text{CH}_3\text{CH}_2\text{OH}+2\text{Na} \rarr 2\text{CH}_3\text{CH}_2\text{ONa} + \text{H}_2
Alcohols will also be oxidised by oxygen. These reactions will produce molecules from another homologous series called carboxylic acids.
2\text{CH}_3\text{CH}_2\text{OH} + \text{O}_2 \rarr 2\text{CH}_3\text{CHOOH}
Fermentation
Ethanol is one of the most commonly used of the alcohol series. It is used to produce alcoholic drinks, as fuels in small stoves, and as antiseptic sprays. It can be produced in a natural process called fermentation.
In fermentation, sugars are broken down by yeast enzymes in anaerobic respiration to produce ethanol and carbon dioxide:
\text{Sugar}\rarr \text{Ethanol} + \text{Carbon Dioxide}
Yeast is a living organism and so requires a certain set of conditions to effectively breakdown the sugar. To work best, the yeast requires a temperature of 37\degree \text{C} and a slightly acidic environment. These conditions are required to ensue that the enzymes in the yeast are able to work. If either the temperature or \text{pH} are too high or too low, the enzymes will denature and will no longer be able to break down sugar.
In addition to these, yeast also requires anaerobic conditions. This means that there should be no oxygen present in the reaction.
Alcohols Example Questions
Question 1: Name a process responsible for producing ethanol.
[1 mark]
Fermentation
(Allow hydration of ethene.)
Question 2: Give the conditions needed for fermentation to occur.
[3 marks]
- 37\degree \text{C}
- Slightly acidic environment.
- Anaerobic (no oxygen).
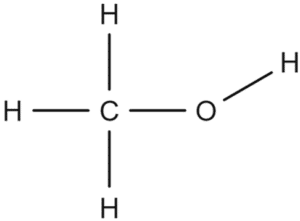
Question 3: Give the structure of methanol.
[1 mark]
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now

