States of Matter
States of Matter Revision
States of Matter
There are three states of matter: solids, liquids and gases. The particle model shows how particles in each of these states are arranged and behave. Each of the different states consist of particles with different arrangements and energies.
Solids, Liquids and Gases
The three states of matter are solids, liquids and gases. For example, we know water can be in each of these states of matter; ice, water, and water vapour. The particles are the same in each of the states, and the only difference between them is their arrangement and energy.
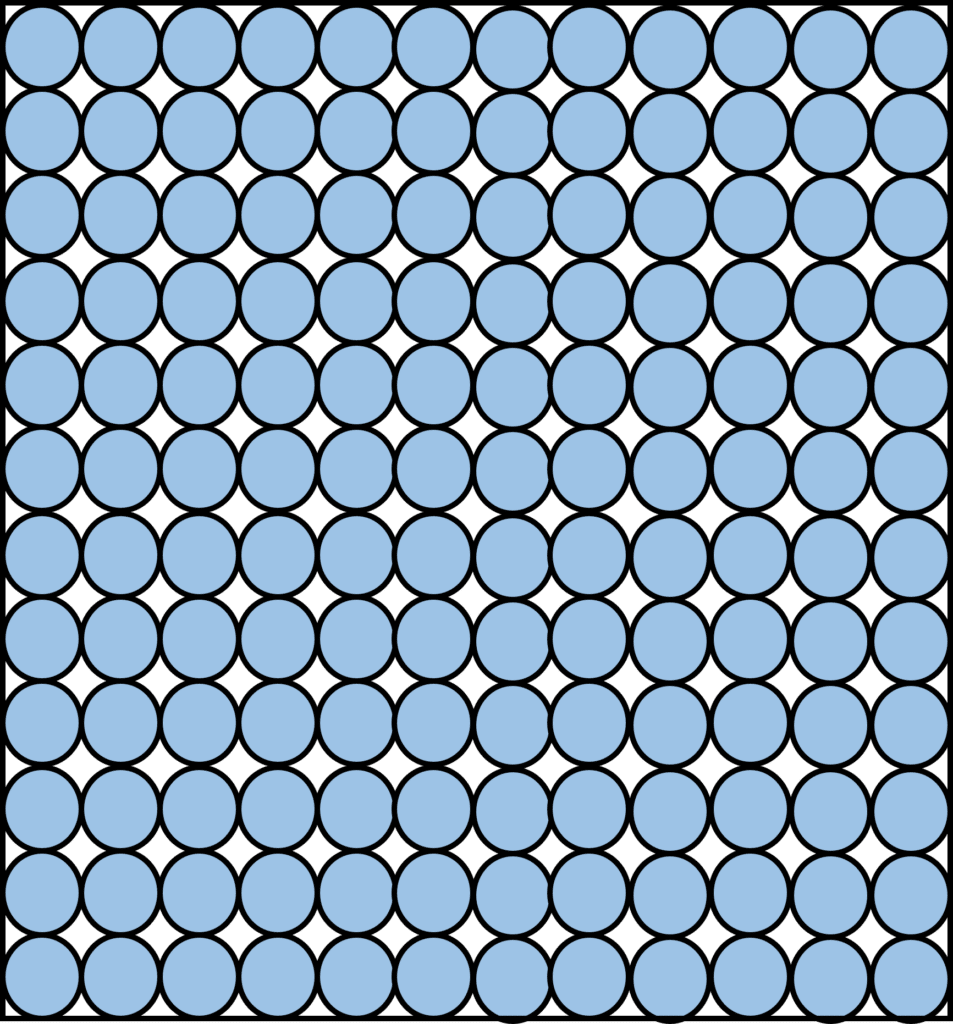
Solids
Particles in a solid have strong forces of attraction between them. As can be seen in the diagram on the right, they are arranged in a close, regular and fixed pattern. The particles have little energy, and so they can only vibrate around the fixed points where they are. Solids tend to have the highest density.
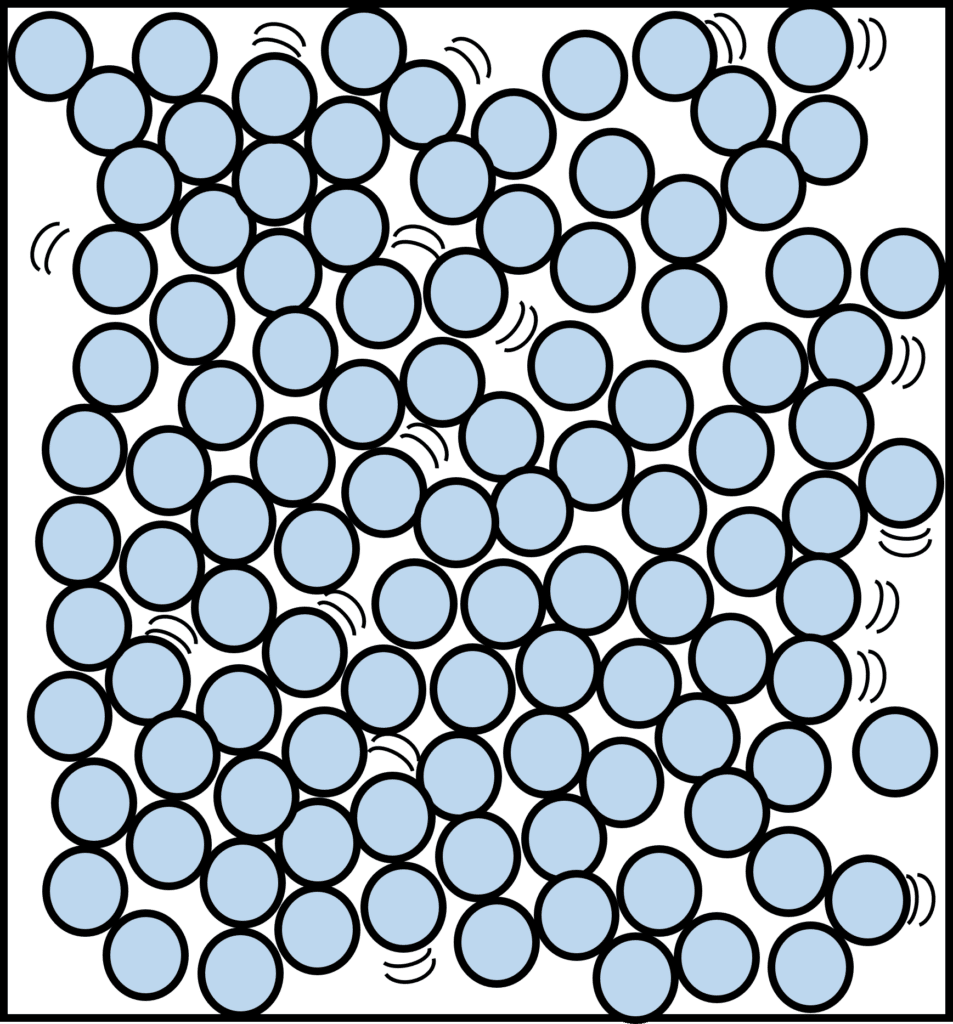
Liquids
In liquids, the forces of attraction between the particles are weaker, and so the particles are free to move around. They are still packed relatively close, but they can move around each other. Therefore, they’re arranged in a more irregular pattern. The particles also have more energy than they do in a solid. Liquids are less dense than solids.
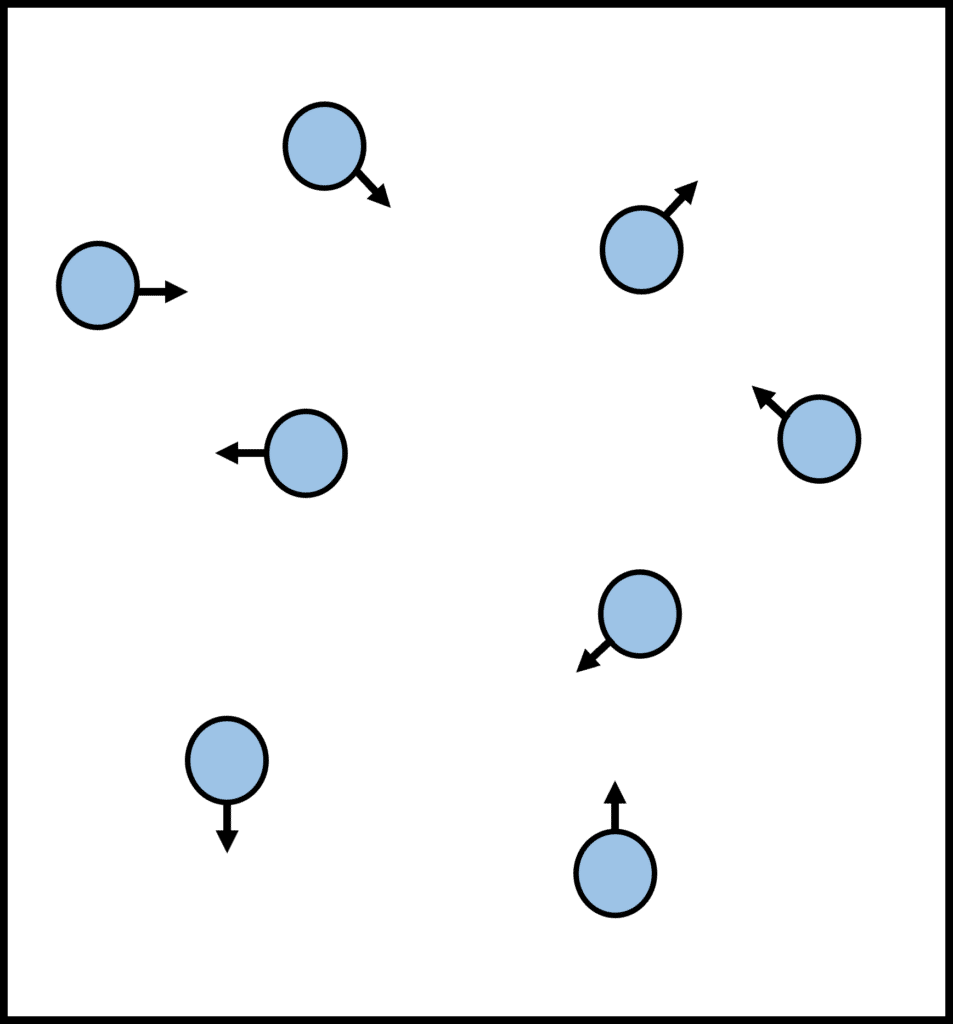
Gases
In gases, the particles are the most spaced out. There are (almost) no forces of attraction between the molecules and so they move freely and in random paths. They have more energy than solids and liquids, and are the least dense of the three states of matter.
Internal Energy
Internal energy is the energy stored inside a system by the particles that make up the system. Particles in a system vibrate or move around. Therefore, they must have a kinetic energy store. The particles also have potential energy because of their position and the bonds between them.
The total kinetic energy and potential energy in a system is the internal energy.
\textcolor{f95d27}{E_{\text{internal}} = E_{\text{k}} + E_{\text{p}}}
The internal energy of a system can change. If you were to heat a system (such as a cup of water), the heat transfers energy to the particles in the system. This increases the kinetic energy of the particles, and so the internal energy also increases. Heating of a system can increase the temperature or change the state of the substance.
Changes of State
If a system is heated enough, it will change state. For example, when ice is heated it melts and becomes water, which is a liquid. Also, if a system is cooled enough, it will change state. For example, if you freeze water it becomes ice, which is a solid.
All the changes of state you need to know are:
- Melting: A change from solid to liquid
- Freezing: A change from liquid to solid
- Boil/Evaporate: A change from liquid to gas
- Condense: A change from gas to liquid
- Sublimate: A change from solid to gas
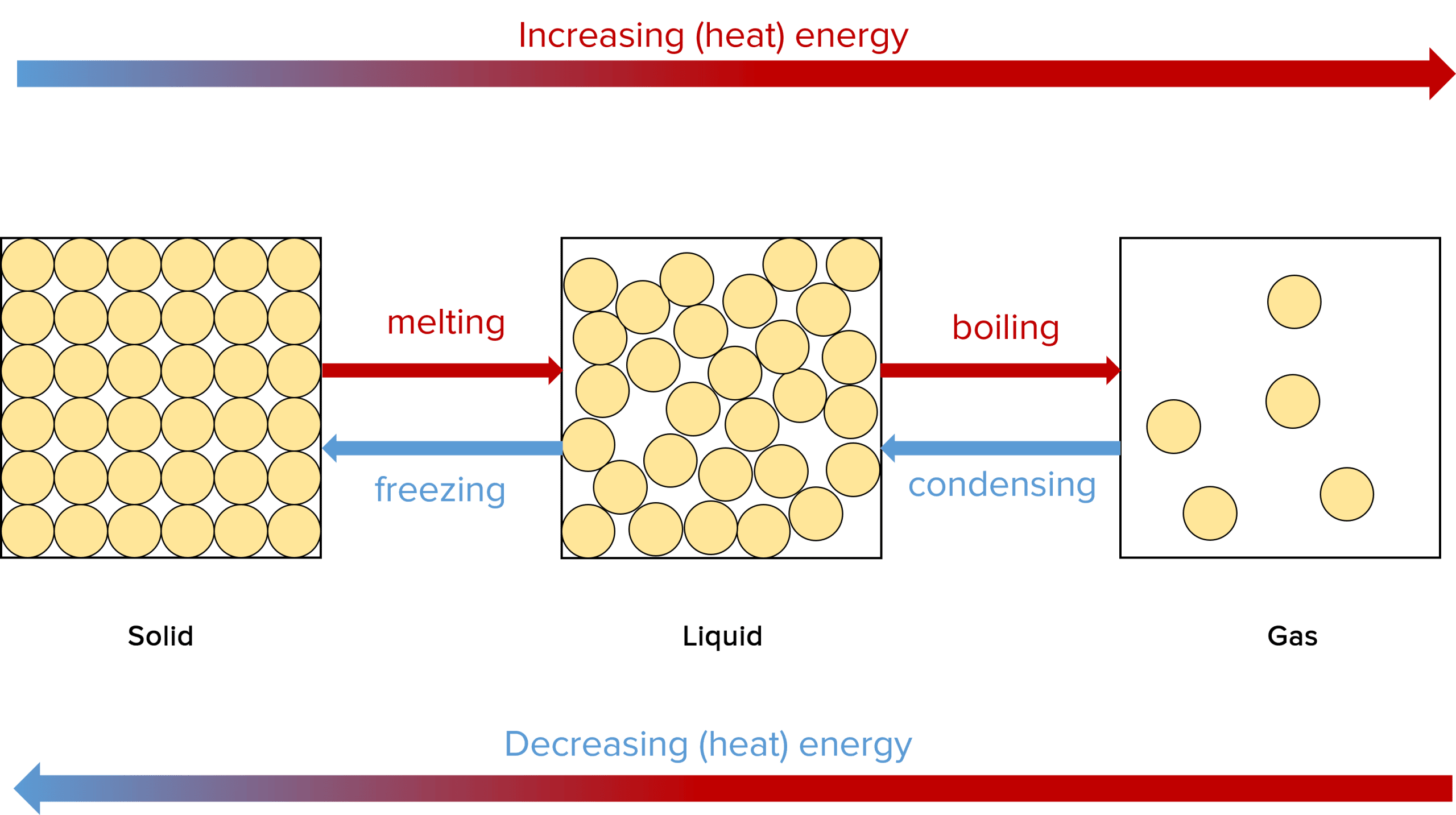
In all of these changes of state, mass is conserved. This is because the number of particles in the system will stay the same, and so the mass of the system will stay the same.
States of Matter Example Questions
Question 1: Explain why particles in a liquid can move past each other.
[2 marks]
There are weaker forces of attraction between the particles, so they are in an irregular pattern and can move past each other.
Question 2: Describe how heating a system would change the internal energy.
[2 marks]
The particle’s kinetic energy increases because heat energy is transferred to them. Therefore the internal energy increases.
Question 3: What is the name of the process where the state of the system changes from a liquid to a gas?
[1 mark]
Evaporation (or boiling)
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now




