Pure Substances and Formulations
Pure Substances and Formulations Revision
Pure Substances and Formulations
Substances can be pure in the everyday sense and in the chemical sense. Substances that are chemically pure can be analysed using melting point and boiling point experiments. Mixtures of substances can be designed for specific purposes. These mixtures are known as formulations.
Purity
When chemists talk about purity, they mean something quite different from the everyday term. When most people talk about purity, they simply mean substances that are unadulterated, i.e. that have had nothing added to them and are in their natural states.
For example, when we talk about a glass of pure water in the everyday sense, we simply mean a glass without contaminants such as bacteria, dirt, or unwanted chemicals. This glass of water will contain other substances apart from the \text{H}_2\text{O} molecule, such as dissolved ions.
For a chemist however, this water would be far from pure. In chemistry, pure substances are defined as ones containing only one compound or element. For a glass of water to be chemically pure, it must contain water molecules, and nothing else.
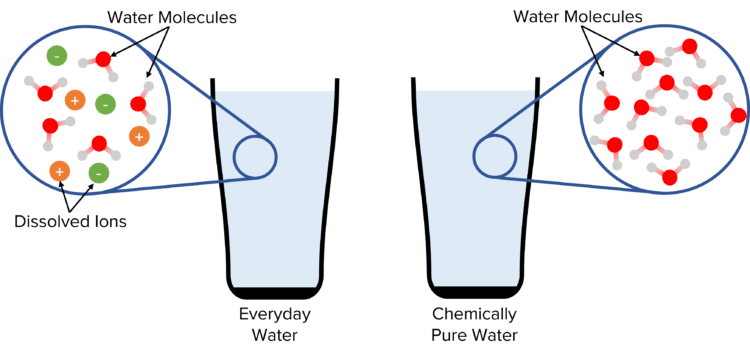
Testing for purity
Chemists are often concerned establishing the purity of substances, particularly in the pharmaceutical industry. There are two quick ways to establish the purity of a substances, both involving changes of state.
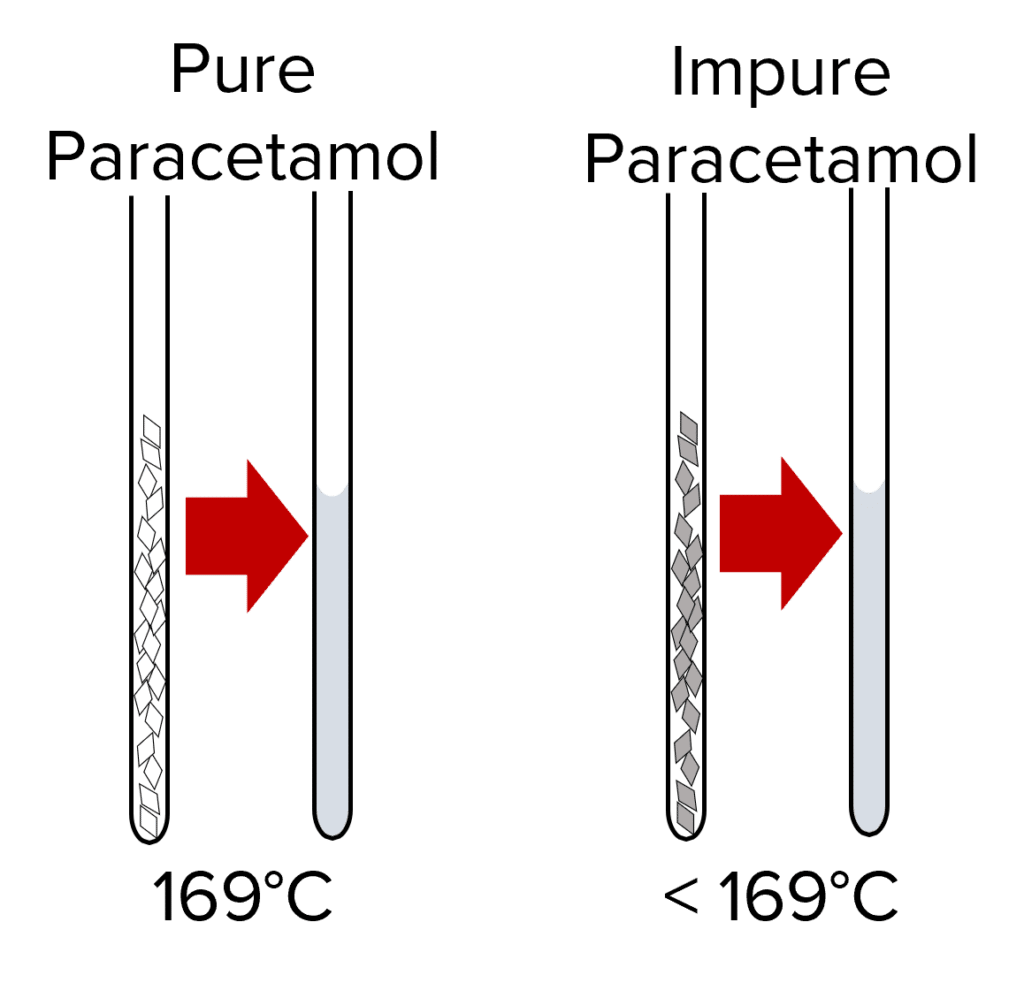
The first is a technique called melting point analysis. In this method, crystals of a solid substances are added to a small vessel called a capillary tube. This capillary tube is placed inside a melting point device.
This machine gradually increases the temperature of the sample until it is hot enough to melt. A small viewing window at the front of the machine allows for the sample to be observed and for the temperature at which it melts to be recorded.
Pure substances will melt exactly at their melting point, where as impure ones will melt below the melting point of the substances and over a range of temperatures.
The second technique is boiling point analysis. In this method, the temperature of a liquid is increased and the temperature at which it starts to boil is measured. Pure substances will boil exactly at their boiling point, but impure ones will boil at a higher temperature over a wider range.
Formulations
Formulations are useful mixtures designed for specific purposes. They are often complex mixtures of chemicals that contain a range of different substances, each chosen to meet a specific need within the formulation.
To produce a formulation, chemicals are mixed in determined quantities to ensure that the right properties are created. If one or more components of the formulation are added in the wrong quantity, the properties of the product will change, leading to less useful and potentially dangerous substances being produced.
Paint is a common example of a formulation. It is made up of a pigment, a solvent, a binder, and additives.
- The pigment is chosen to give the paint its colour. Most of the time this is a metal oxide, such as titanium oxide for white paints.
- The solvent is used to dissolve the other components of the formulation to produce a solution and alter the viscosity of the paint. This component will then evaporate off when the paint is applied.
- The binder is used to stick the pigment to whatever is being painted.
- The additives are added to give the paint any other desired properties, such as a gloss or matt finish, or to achieve certain behaviours like glowing in the dark.
Other common examples of formulations are medicines, soft and alcoholic drinks, metal alloys, cleaning products, and a whole host of other industrially produced chemicals.
Pure Substances and Formulations Example Questions
Question 1: State the difference between a substance that is pure in the everyday sense of the word vs. a substance that is pure in the chemical sense of the word.
[2 marks]
- Substances that are pure in the every day sense are free of additives.
- Substances that are pure in the chemical sense of the word contain only one compound or element.
Question 2: State the definition of a ‘formulation’.
[2 marks]
A chemical mixture designed for a specific purpose.
Question 3: Give two examples of formulations.
[2 marks]
Any two from:
- Paint
- Medicine
- Soft Drinks
- Alcoholic Drinks
- Cleaning Products
Question 4: Ibuprofen melts at a temperature of 157\degree\text{C}. A sample known to contain impurities is analysed using a melting point device. Describe the results you would expect from this analysis.
[2 marks]
The sample would melt at a lower temperature and would likely melt over a range of temperatures.

MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



