Chromatography
Chromatography Revision
Chromatography
Chromatography can be used to separate mixtures into their component compounds. It can also be used to identify the different compounds present in a mixture. By calculating \text{R}_f values, paper chromatography can be used to identify different chemicals from a mixture.
Chromatographic Methods
Chromatography refers to a group of methods that can be used to separate mixtures of chemicals into their component parts. There are lots of different methods of chromatography but all methods have three components:
- The analyte: The mixture of compounds being separated during the chromatography experiment.
- The stationary phase: A polar substance (often solid) through which the mobile phase moves.
- The mobile phase: A solvent used to dissolve the analyte. The mobile phase moves through the stationary phase, carrying the analyte with it.
During a chromatography experiment, the analyte moves through the stationary phase which will attract the components of the mixture to itself. The strength of this attraction will depend on the polarity of the stationary phase and the different polarities of the analyte components. The speed with which a compound moves through the stationary phase is dependent on the strength of its attraction to the stationary phase.
Compounds with a strong attraction to the stationary phase will be slowed down by this attraction and so will take a long time to move through the system. Compounds that experience a weak attraction to the stationary phase will move through the system more quickly.
The difference in the time taken for compounds to move through the stationary phase will lead to their separation.
Paper Chromatography
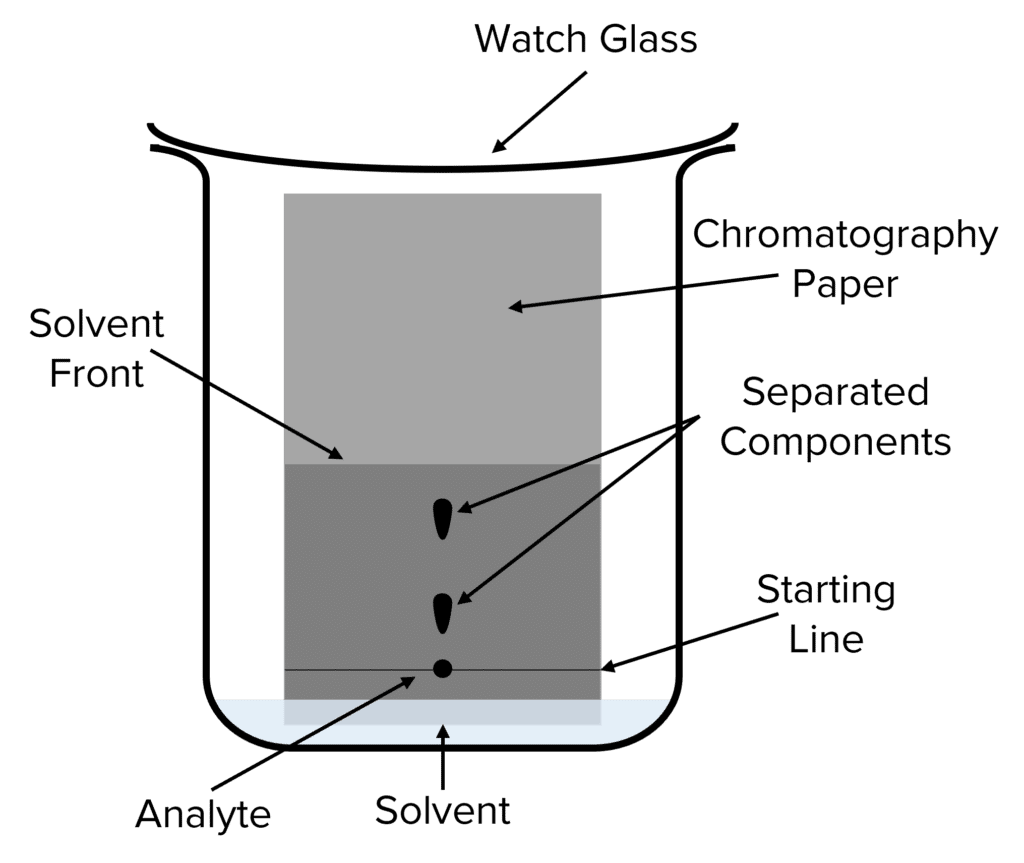
Paper chromatography is a type of chromatography that is often used to quickly analyse mixtures in chemistry. This method employs chromatography paper as the stationary phase and a solvent as the mobile phase. During paper chromatography the solvent soaks into the chromatography paper creating a line called the solvent front. This solvent front moves up the length of the chromatography paper, pulling the analyte up along with it.
As the analyte moves up the paper, the attractions between its components and the stationary chromatography paper separates them out. Compounds that are more polar (i.e. experience stronger electrostatic attractions) will move a small distance up the paper before they become stuck, compounds with a weaker attraction will move a greater distance.
The distance that components move up the chromatography paper can be quantified by calculating \text{R}_f value. This value is calculated by diving the distance traveled by a component by the distance traveled by the solvent:
\text{R}_f=\frac{\text{Distance Traveled by Component}}{\text{Distance Traveled by Solvent}}
The \text{R}_f value of a given compound remains constant, it will move the same distance up the chromatography paper relative to the solvent. We can identify compounds in mixtures by calculating their \text{R}_f values.
Required Practical
Chromatography Paper
Paper chromatography can be used to show the different components that make up black ink, usually a mixture of different coloured chemicals.
Method
- With a ruler and pencil, draw a straight line across a rectangle of a chromatography paper 2\text{ cm} in from the short edge of the rectangle. Note, you must use a pencil for this as a line drawn in ink will dissolve in the solvent.
- Pour solvent into a beaker to a depth of 1\text{ cm}.
- Using a pipette drop a sample of ink on to the middle of the pencil line and draw a circle around it in pencil.
- Place the chromatography paper into the beaker so that the starting line is just above the solvent. Ensure that the ink spot does not fall below the solvent surface.
- Place a watch glass over the top of the beaker and let the solvent front move up the chromatography paper. When it is about 1\text{ cm} from the top of the paper, remove the paper from the beaker.
- Using a pencil, draw a line across the solvent front and circle the spots left by the separating ink.
Analysis
- Using a ruler, measure the distance between the starting line of the experiment and the solvent front.
- Measure the distances that each ink spot has traveled from the starting line.
- Calculate the \text{R}_f values for each spot by dividing the distance traveled by the spot by the distance traveled by the solvent front.
Note: when measuring the distance traveled by a spot across the paper, remember to measure from the centre of the spot.
Chromatography Example Questions
Question 1: Define the term ‘stationary phase’.
[2 marks]
A polar solid substance through which the mobile phase moves.
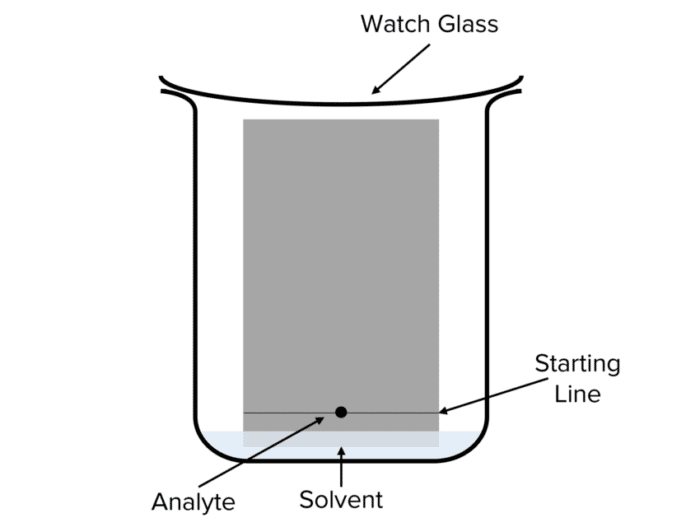
Question 2: A student carries out a paper chromatography experiment. Below is an image of their setup:
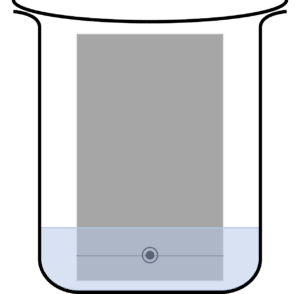
State an issue with the students set up.
[2 marks]
Too much mobile phase has been added, submerging the ink spot.
This will cause the ink spot do dissolve into the mobile phase before the experiment is completed.
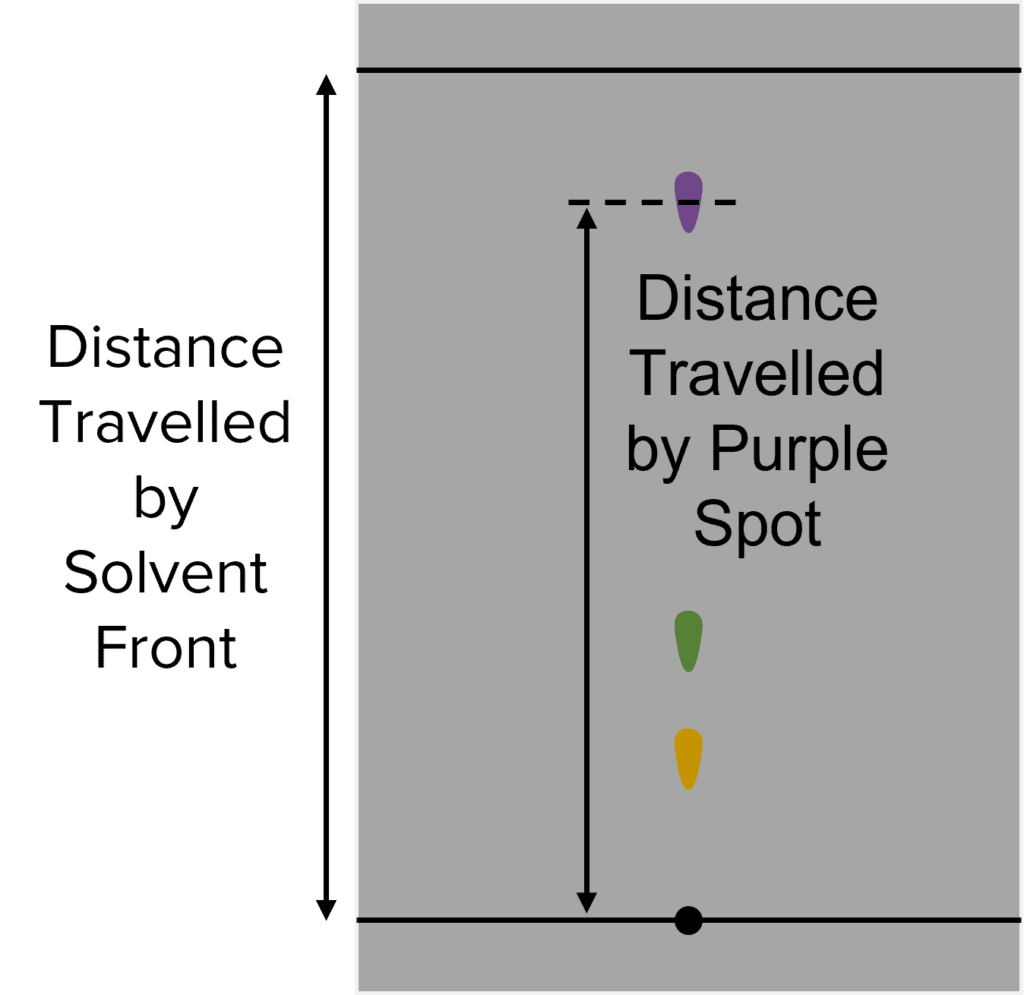
Question 3: In another experiment a student produces the following chromatography paper:
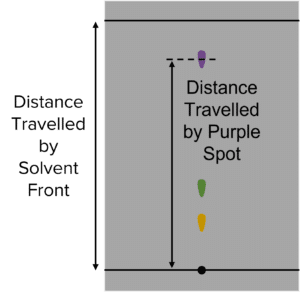
Calculate the \text{R}_f value of the purple spot.
[2 marks]
\text{R}_f=\frac{\text{Distance Travelled by Purple Spot}}{\text{Distance Travelled by Mobile Phase}}\\\text{}\\=\frac{4.67}{5.23}\\\text{}\\=0.89
Chromatography Worksheet and Example Questions
Purity, Formulations and Chromatography Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



