Polymers
Polymers Revision
Polymers
Polymers are a class of molecule made up macromolecules. They have a number different uses in every day life and can be made in different ways. Polymers can be both synthetic and natural. The formulae of polymers are constructed in a different way to other hydrocarbon molecules.
What are Polymers?
Polymers are a special class of hydrocarbon molecule. Unlike the majority of the families of molecules so far studied, polymers do not exist as simple molecules. Instead, polymers exist as long chains of primarily carbon. The chains can range from a few dozen carbon atoms long, all the way up to tens of thousands of atoms long.
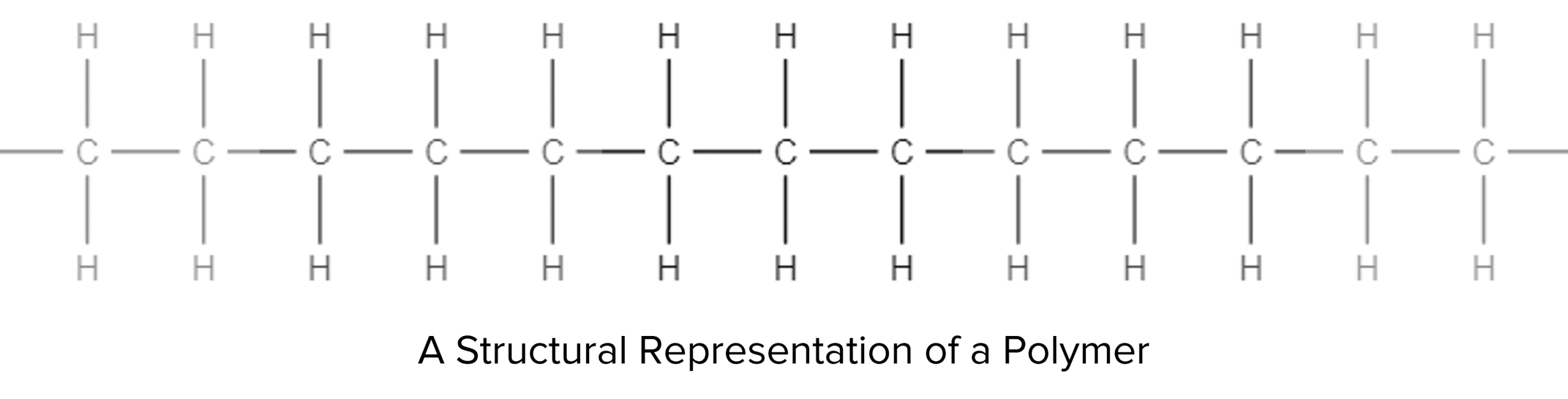
Polymers are made up of repeating units called monomers. These monomers will bond together to form long chains. A monomer will typically be a small hydrocarbon molecule with a functional group, for example, the polymer above is made out of a repeating monomer of ethene:
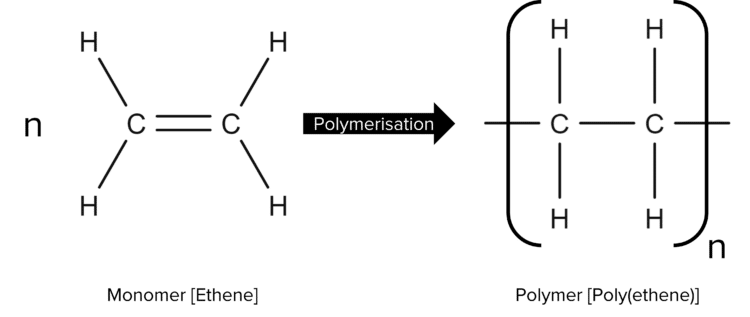
Because polymers can be thousands of monomer units long, it is not practical to draw out their structures every time they are discussed. Instead we use the representation shown in the above diagram. There are 3 steps to drawing the structure of a polymer.
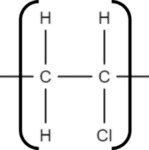
1. Identify the repeating unit of the polymer. Take for example poly(vinyl chloride) (PVC):
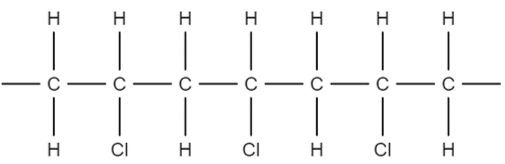
We can see that the structure repeats every two carbon atoms. This is the simplest repeat unit of the polymer.
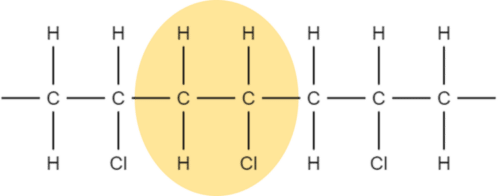
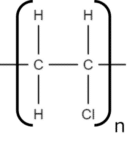
2. Draw out the repeat unit and bracket it:
3. Note the number of repeat units in the polymer chain. If the number is known then it should be written at the bottom right of the polymer repeat unit outside the bracket. If the number is not known, we use the letter n instead:
Addition Polymerisation
Addition polymerisation is a common method used in the formation of polymers from alkene monomers. In addition polymerisation, the double bond of an alkene monomer is opened up, allowing it to bond with another alkene monomer. This will create a chain of monomers, with each new monomer molecule added increasing the length of the chain. By repeating this process again and again, a polymer chain is built up.
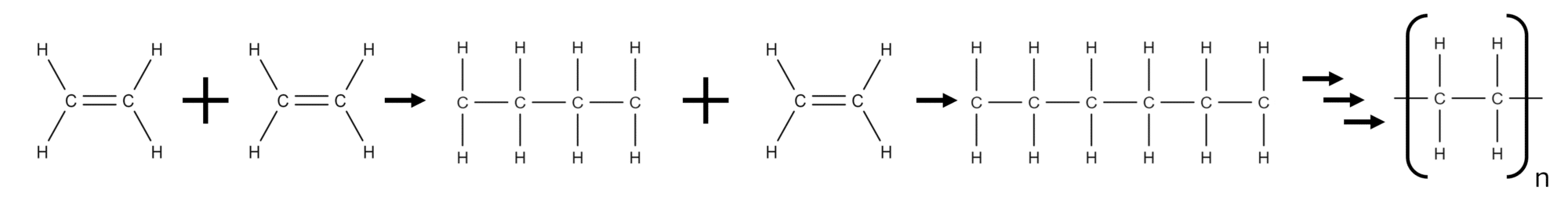
When formed via addition polymerisation, all the atoms of the monomer molecules can be found in the polymer chain. In addition polymerisation the polymer is the only product so no atoms are lost to side products.
Condensation Polymerisation
Unlike addition polymerisation, which involves monomers with the same functional groups, condensation polymerisation involves monomers with different functional groups. For example, condensation polymers will be formed when a di-alcohol (a molecule with two alcohol functional groups) and a di-acid (a molecule with two carboxylic acid functional groups).
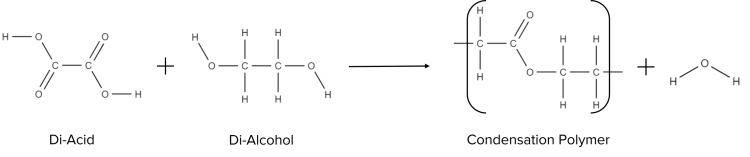
Unlike addition polymerisation, condensation polymerisation will yield two products, one large polymer chain and one simple molecule, often water. For example, in the polymerisation reaction given above, for each new bond formed, one water molecule is also produced. This happens because, upon reaction, the \text{OH} group of the acid breaks away and removes the \text{H} atom from the alcohol group.
In most cases, condensation polymerisation will involve two molecules that each contain a pair of functional groups that are the same. In some cases however the molecules involved are more complex. This is the case for the natural polymers like DNA.
Amino Acids
Amino acids are naturally occurring molecules that contain both a basic amino \left(\text{--NH}_2\right) group and an acidic carboxylic acid \left(\text{--COOH}\right) group.
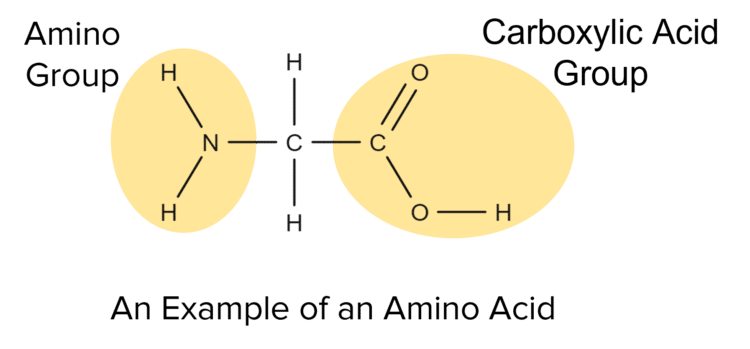
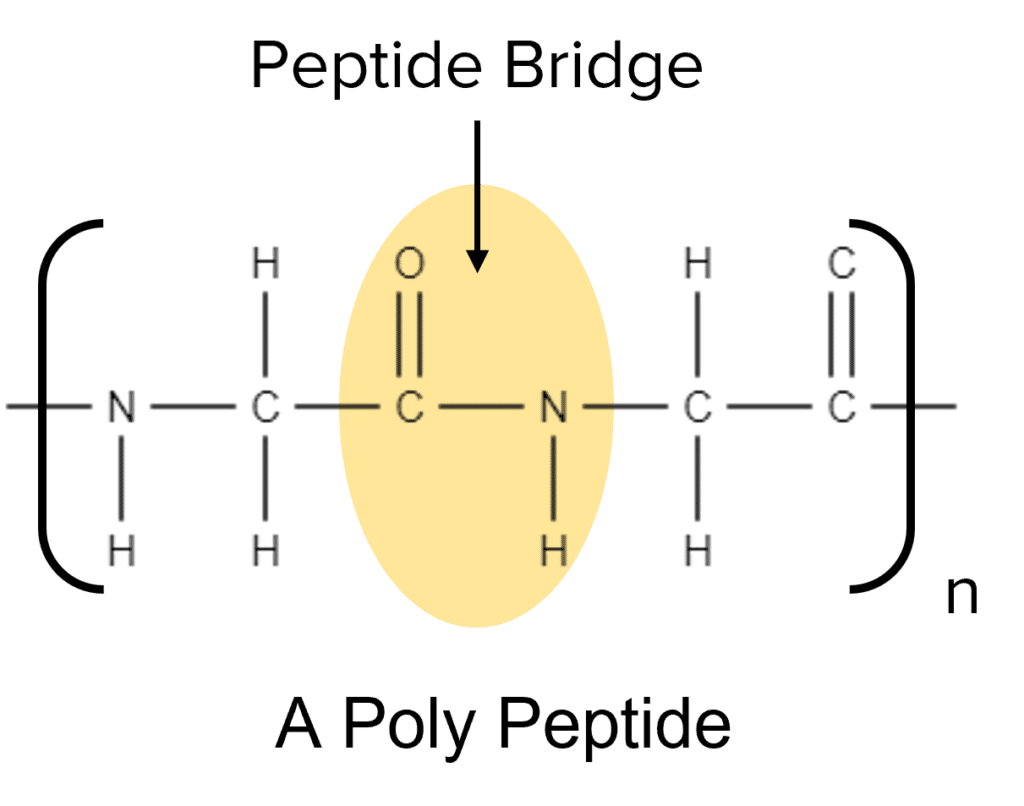
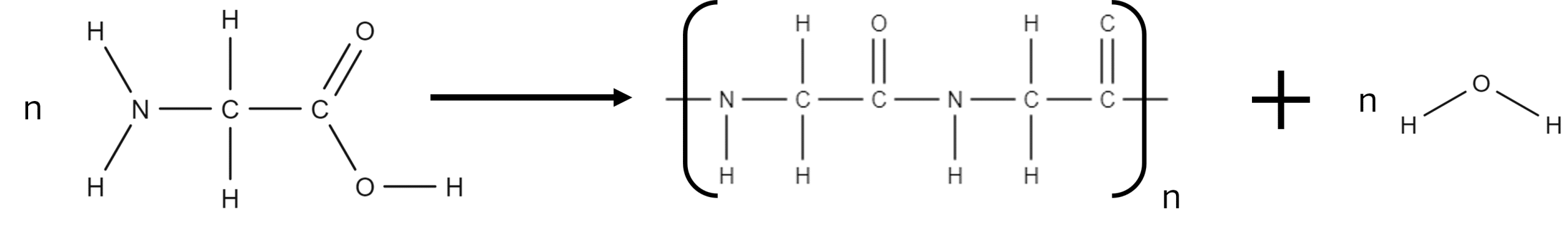
Among the interesting properties of amino acids is their ability to react with themselves. Amino acids are able to undergo condensation polymerisation with themselves to form a special type of natural polymer called a polypeptide. Polypeptides get their name from the peptide bridges (peptide bonds) they contain.
A peptide bridge is a functional group consisting of a carbon double bonded to an oxygen and single bonded to a nitrogen atom, which is itself bonded to another carbon atom on the other side. Peptide bridges connect organic molecules together, building up large polymer chains.
Polypeptides can consist of repeating chains of the same amino acid or of different amino acids arranged in different patterns. Long combinations of different amino acids form natural proteins found in living matter.
Since polypeptides are formed from condensation reactions, there will be an additional product in the form of a molecule of water.
DNA
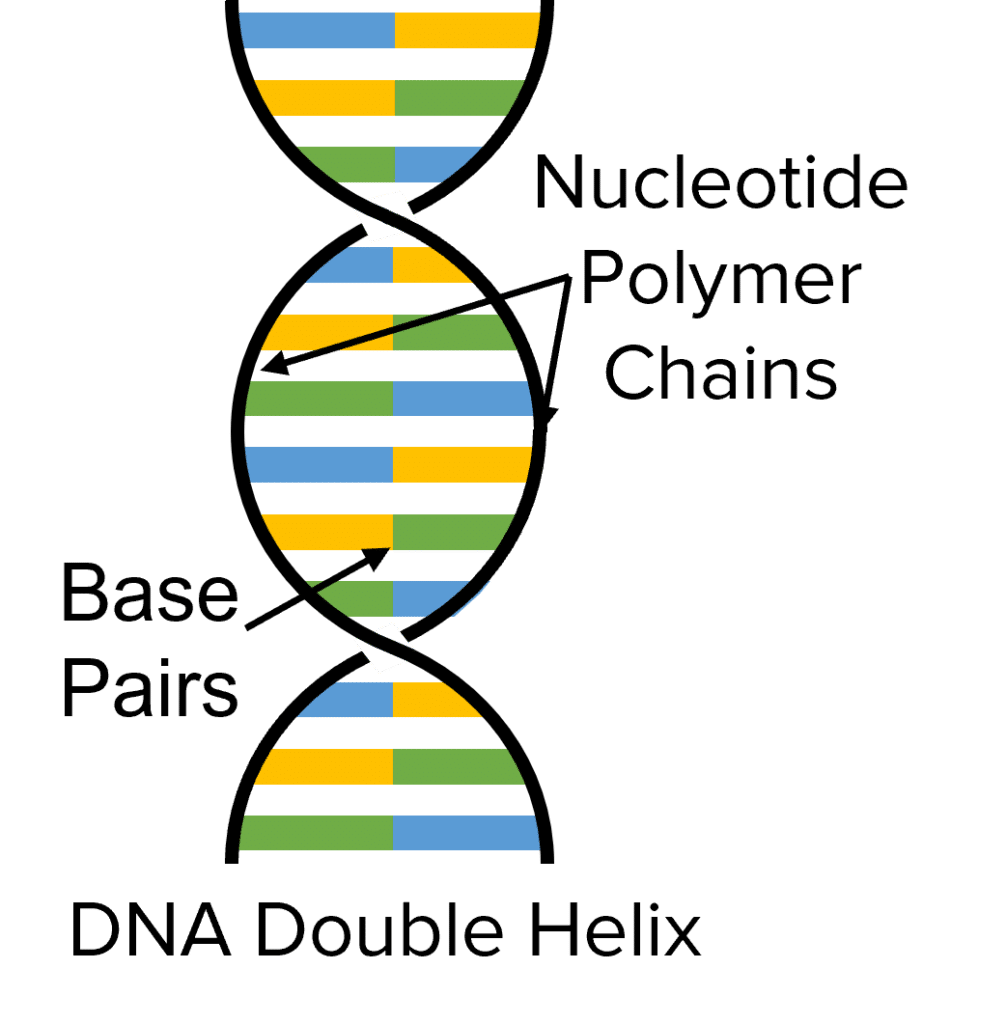
DNA (Deoxyribonucleic Acid) is another example of a natural polymer. DNA is essential for all life on earth since it tells living things how to grow and develop.
DNA is formed of two long polymer chains, built out of the combination of four types of monomers called nucleotides. These two polymer chains are wrapped around each other in a structure known as the double helix (discovered by Rosalind Franklin in 1953).
The two strands of the helix is held together by links between base pairs. The sequence of these pairs contain the code used by living organisms to control the building of proteins. Other examples of natural polymers include starch and cellulose.
Polymers Example Questions
Question 1: Describe the difference between addition polymerisation and condensation polymerisation.
[4 marks]
- Condensation polymerisation happens between monomers with different functional groups.
- Condensation Polymerisation produces two products.
- Addition polymerisation happens between alkene monomers.
- Addition polymerisation only produces one product.
Question 2: Give the structure of the monomer of polyvinyl chloride.
[1 mark]

Question 3: State the role of DNA in living organisms.
[1 mark]
DNA controls the building of proteins.
Question 4: Describe the differences between condensation polymerisation and addition polymerisation.
[4 marks]
- Condensation polymerisation happens between monomers with different functional groups.
- Addition polymerisation happens between monomers containing alkene functional groups.
- Condensation polymerisation produces two products, a polymer and a simple molecule.
- Addition polymerisation produces only one product, a polymer.
Question 5: Who discovered the structure of DNA?
[1 mark]
Rosalind Franklin (allow Watson and Crick)
Polymers Worksheet and Example Questions
Synthetic and Naturally Occurring Polymers Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now

