Covalent Bonding
Covalent Bonding Revision
Covalent Bonds
Covalent bonds are strong bonds that form between two or more atoms that are both non-metal elements. The name covalent comes from the idea that the atoms in a covalent molecule share their outer (known as valence) shell electrons, hence co-valent. Atoms in a covalent molecule will often have full outer shells thanks to this electron sharing. This makes covalent molecules very stable, and covalent bonds very strong (even stronger than ionic bonds). Covalent compounds can either be molecules (e.g. \text{H}_2\text{O}), larger molecules (e.g. polymers), or even special structures know as giant covalent structures (e.g. Diamond).
Formation of Covalent Bonds
Covalent bonds form when two or more atoms of non-metal elements come together to form a molecule. These may be atoms of the same non-metal element, or of different non-metal elements. All the atoms will have an almost full outer shell that they want to fill. Unlike ionic bonding, it is not favorable for any of the atoms to lose electrons. Instead, the most effective way for these non-metal atoms to gain a full outer shells is for them to share one or more pairs of electrons between each other. This can be illustrated by the \text{H}_2 molecule:
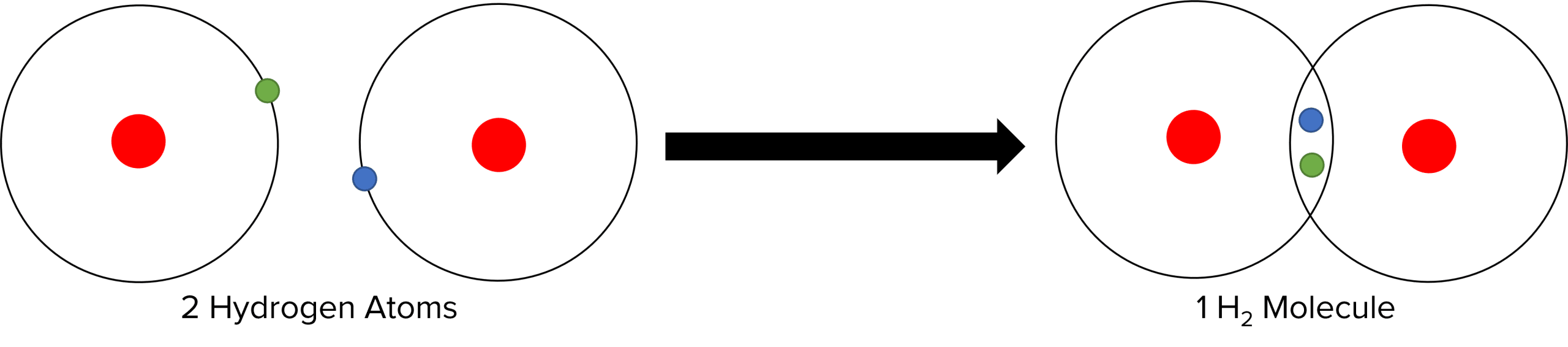
Each hydrogen atom has a half filled shell. To get a full outer shell, each atom needs to gain one electron. They are able to do this by sharing a pair of electrons between each other, with each atom donating one electron to the bond. These bonds are very strong and stable and as such covalent molecules are often very hard to break apart. As neither atom is losing nor gaining electrons they remain neutral in a covalent bond.
Drawing Covalent Bonds
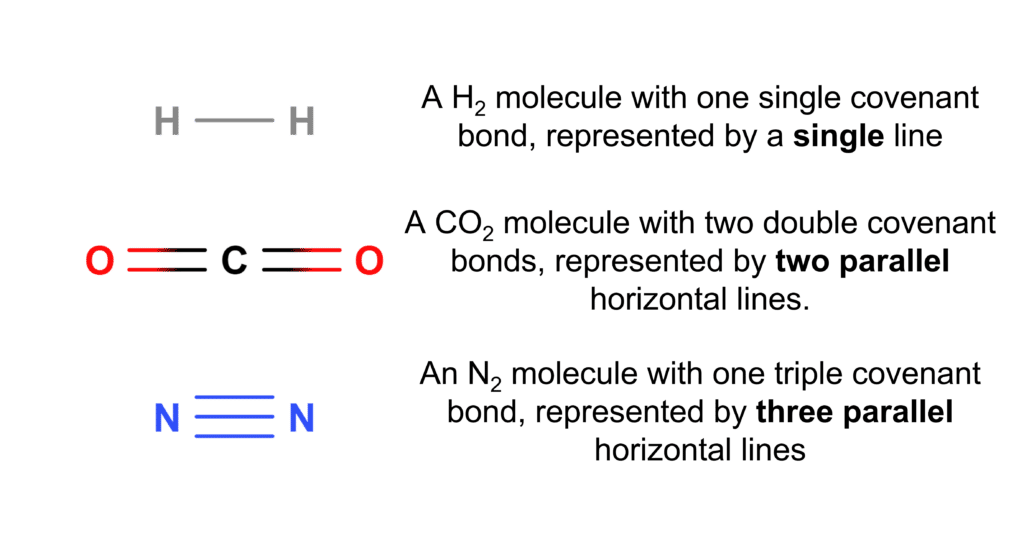
Covalent bonds can be represented in a few different ways. The most common way to use a line to represent a single covalent bond. In cases where there is a double or triple covalent bond, as in \text{CO}_2 or \text{N}_2, a set of parallel lines are used, with the same number of lines as there are bonds (e.g. a triple bond is represented by three parallel lines) . This is illustrated in the diagram to the right.
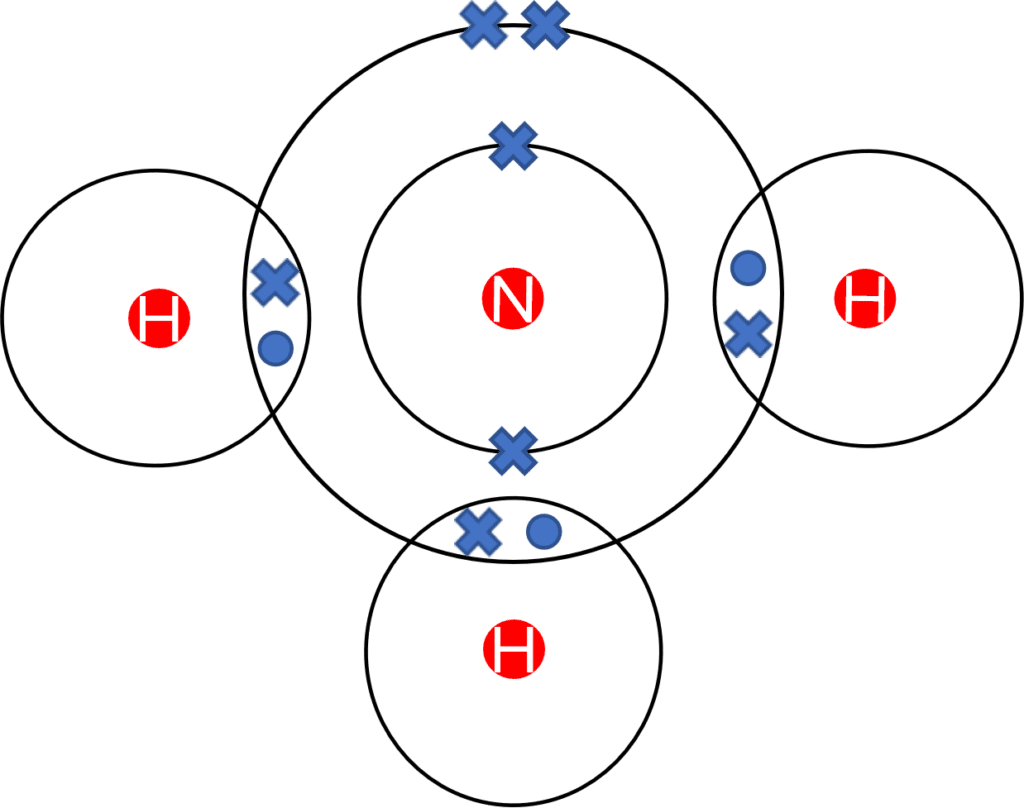
Alternatively, Dot and Cross diagrams may also be used to represent a covalent bond. Covalent dot and cross diagrams operate in the same way as those for ionic molecules. However, in the case of covalent molecules, the diagrams look more like a Venn diagram, with the dot and cross of the covalent bonds contained within the overlap. The diagram to the left shows a dot and cross diagram of ammonia \left(\text{NH}_3\right), a common covalent molecule.
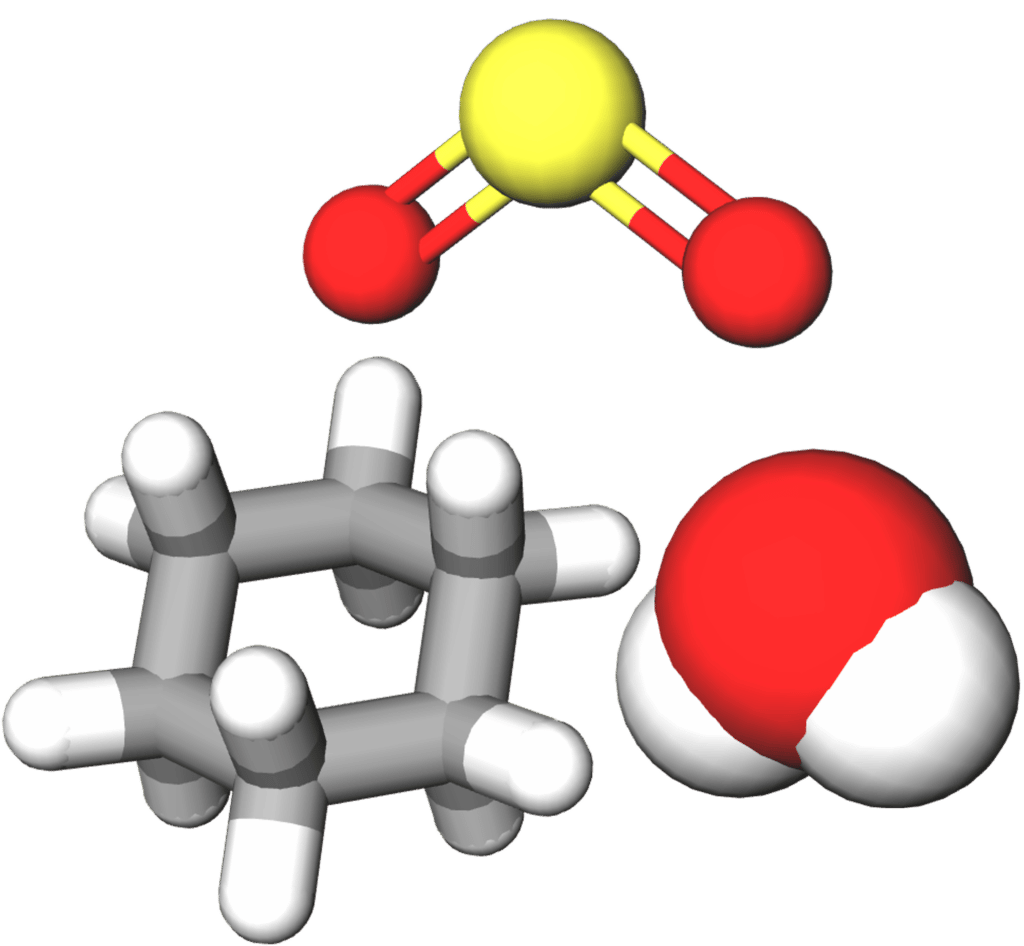
Lastly, modern computing techniques mean that covalent molecules can be represented by 3\text{-D} models. These models can be used to show a range of different aspects of a a molecule, and can often look very different deepening on what the researcher wants the model to display.
Each of the above representations has their limitations. Dot and cross diagrams allow us to visualize how electrons are shared through out a molecule and from which atoms they originated, but do not tell us anything about the shape of those molecules. Line diagrams can tell us more about the 2\text{D} shape of a molecules (e.g. the angles between bonds) but fail to represent the space filled by atoms and are unable to represent them in 3\text{D}. Three dimensional models can show more accurately the shapes of molecules and the spaces filled by their constituent atoms. However, we usually lose some information, for example the distribution of electrons within the molecule.
Properties of Simple Covalent Molecules
Most covalent compounds exist as simple molecular substances. These substances can have a range of properties but there are some general trends that most of them follow. The bonds between atoms in a covalent molecule are very strong and difficult to break but the forces between molecules are are usually very weak. These weak intermolecular forces are much more easily overcome than the strong electrostatic attractions found in ionic substances. This means that most simple molecular substances are liquids or gasses at room temperature. The smallest molecules will have the lowest boiling points as they have the weakest intermolecular forces.
As molecules increase in size, the intermolecular forces between them increase and as a result, so do their melting and boiling points. This is why, at room temperature, hydrogen (the smallest possible molecule) is a gas, where as \text{H}_2\text{O} is a liquid, and polymers are solids.
The atoms in covalent molecules are not charged as they have the same number of protons and electrons. This means that covalent compounds do not conduct electricity, as there are no charged particles to conduct.
Covalent Bonding Example Questions
Question 1: The most important covalent molecule on Earth is water, made up of one oxygen atom and two hydrogen atoms. Give the dot and cross diagram of a water molecule.
[3 marks]
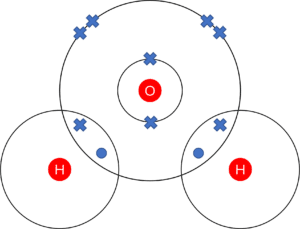
- 1 mark for one oxygen atom and two hydrogen atoms.
- 1 mark for 6 crosses on the outer shell of oxygen. (4 on the outer ring, 1 each in the overlaps)
- 1 mark for 1 dot per hydrogen atom. (1 each in the overlaps)
(note: marks can be gained whether or not inner structure of oxygen is given. Important points are that the outer rings contain the correct numbers of electrons and that the atoms are correctly indicated)
Question 2: Covalent molecules can be represented using line diagrams or 3\text{D} models. Compare the contrast the two methods.
[4 marks]
Key Content:
- Line diagrams can be used to represent the 2\text{D} shapes of molecules, specifically the angles between bonds.
- However line diagrams do not tell us anything about the 3\text{D} shape of a molecule.
- 3\text{D} models can tell us a lot about the three dimensional shape of a molecule, as well as the space occupied by its atoms.
- Neither method tells us much about the distribution of electrons in a molecule.
Question 3: Fluorine \left(\text{F}_2\right) exists as a simple covalent molecule, with a relatively small size. Predict what what state of matter fluorine would be found in at room temperature.
[2 marks]
A fluorine molecule is very small. As such it has very weak intermolecular forces. These forces are easily overcome and so fluorine is a gas at room temperature.
Covalent Bonding Worksheet and Example Questions
Covalent Bonds Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now



