Periodicity
Periodicity Revision
Periodicity
Periodicity is the study of patterns in the chemical or physical properties of elements going across the periods of the periodic table.
The Classification of Elements
Elements in the periodic table can be divided into one of four blocks known as the s, p, d and f blocks. The block that an element is in will depend on the orbital in which the outermost electron, known as the valence electron, of the element is found.
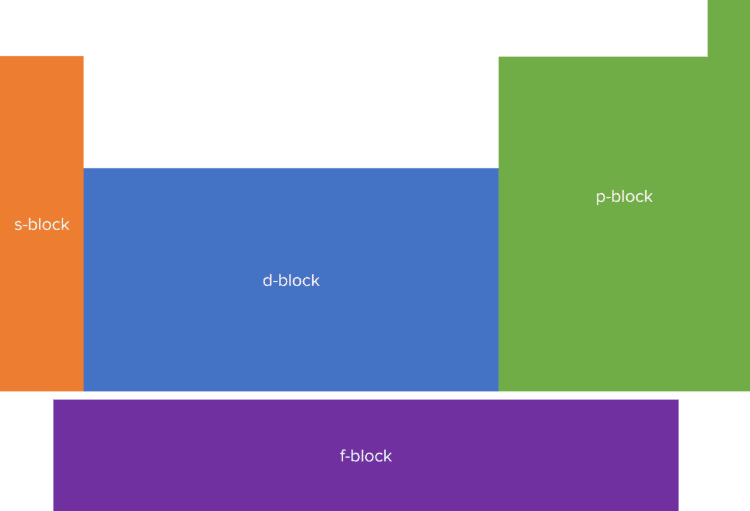
Group 1 and 2 elements are found in the s-block. Elements in the s block of the periodic table will hold their outer electrons in an s orbital. Elements found in groups 3 through 8 are are found in the p-block. These elements hold their outer electrons in one of three p orbitals.
The d-block of the periodic table contains the transition metals. These elements hold their outer electron in one of five d orbitals. Finally, the f-block contains the actinides and the lanthanides. These elements are typically radio active and have outer electrons that can be found in one of seven f orbitals. The f block is not found with the rest of the periodic table but is instead found separated out below.
Trends in Atomic Radius
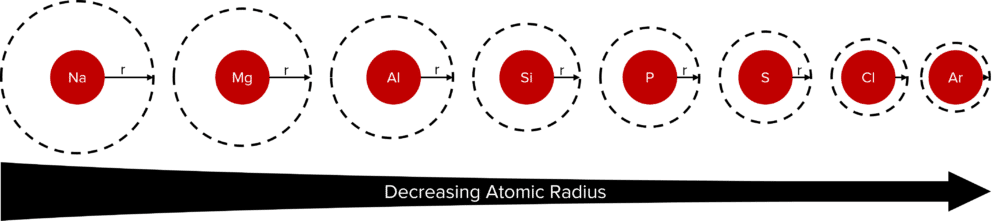
Across a period, going from left to right, the atomic radius decreases. As we go across the period the number of protons found in the each elements nucleus increases. This increases the nuclear charge of the elements, thereby creating a stronger electrostatic attraction between the nucleus and the electrons of the element.
The outer electron of each element in a period is found in the same shell. This means that these valence electrons will experience roughly the same amount of shielding from the other electrons of the element. Shielding is the term given to the electrostatic repulsion felt between negatively charged electrons and how much this cancels out the attraction of electrons to the nucleus. More electron shells means more electron-electron repulsion and so more shielding.
The roughly similar shielding means that, across a period, the nucleus of an element is able to pull its valence electrons closer to itself.
Trends in the First Ionisation Energy
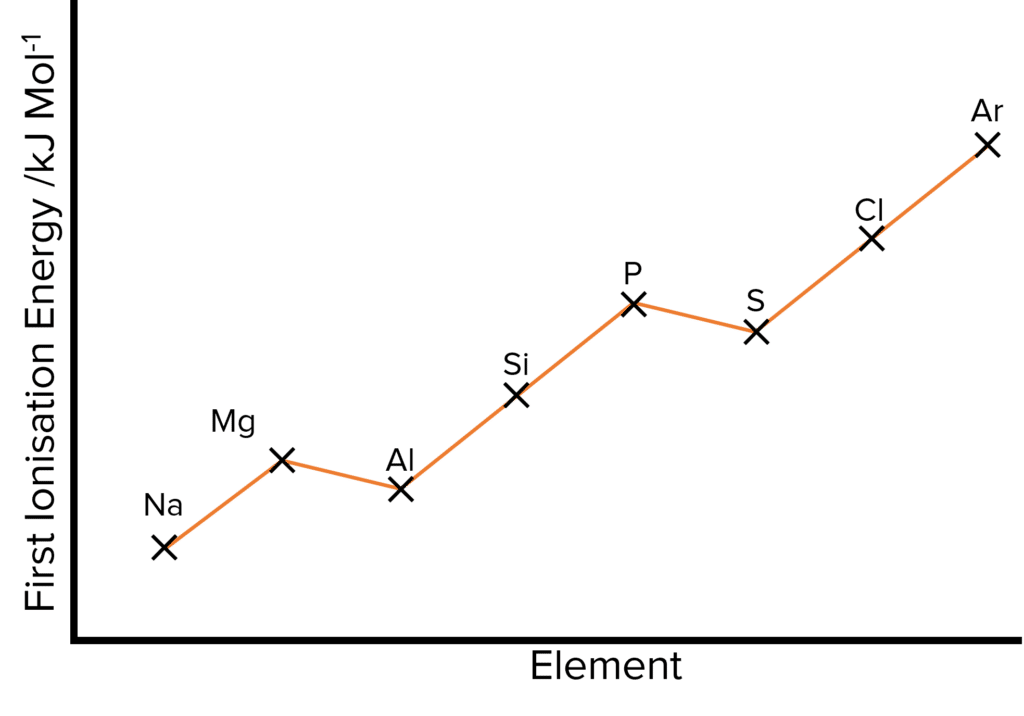

The increasing nuclear charge andattraction that leads to a decrease in atomic radii also leads to an increase in first ionisation energy. This means that more energy is needed to remove this valence electron from the element to form an ion. As the shielding across the period remains similar, there is no significant increase in the repulsion of this outer electron and so the ionisation energy increases.
Exceptions:
Magnesium and Aluminium:
Between magnesium \left(\text{Mg}\right) and aluminium \left(\text{Al}\right), there is a drop in the 1st ionisation energy.
This happens because:
- The outer electron of \text{Mg} is in the 3s subshell while the outer electron of \text{Al} is in the 3p subshell.
- So when removing an electron from the \left(\text{Al}\right) outer shell, the electron is being removed from a 3p subshell which has a higher energy than the 3s subshell.
- The higher energy level makes it easier for an electron to be removed, decreasing the energy required to remove the electron.
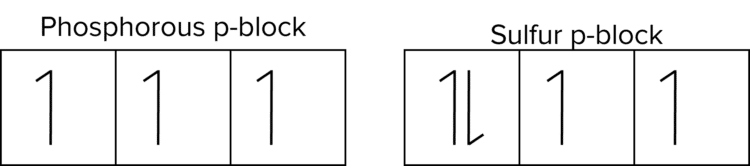
Phosphorous and Sulfur:
There is also a drop in 1st ionisation energy, between phosphorus \left(\text{P}\right) and sulfur \left(\text{S}\right).
This happens because:
- Although the outer electrons in both phosphorous and sulfur are in 3p orbitals, the outer electron of sulfur is paired up with another electron while the outer electron of phosphorous is not.
- This causes a small repulsion between the two electrons making it easier for an electron to be removed.
- It is also more energetically favourable for the atom to have an exactly half full subshell. Sulfur can lose its outer electron to gain an exactly half filled subshell.
KeyNote: The trends for Period 3 are the exact same for Period 2. Except that the drops are between berylium \left(\text{Be}\right) and boron \left(\text{B}\right) and nitrogen \left(\text{N}\right) and oxygen \left(\text{O}\right). In an explanation, make sure to change 3p/3s orbitals to 2p/2s.
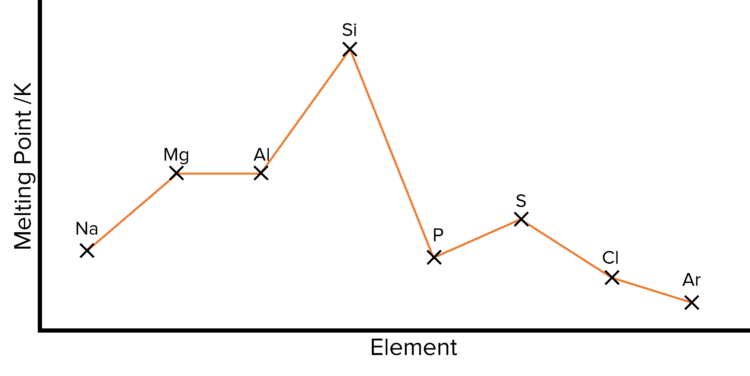
Melting and Boiling Points
There is no general trend in the melting and boiling points of elements across a period. Instead a number of factors determine the individual temperatures at which each elements will melt and boil.
From sodium \text{Na} to aluminium \text{Al} melting/ boiling points increase.
This is because:
- There is an increase in the strength of metallic bonding.
- The metallic bonds become stronger because the number of delocalised electrons increases across the period while the ions become smaller with a greater positive charge.
- To break these bonds, a high energy is required.
Silicon \left(\text{Si}\right) exists a macromolecule and therefore has lots of strong covalent bonds within it. A lot of energy is required to break these bonds so it has a very high melting and boiling point.
Chlorine \left(\text{Cl}\right), sulfur \left(\text{S}\right) and phosphorus \left(\text{P}\right) are all simple molecules that contain weak Van der Waals forces. Therefore, not a lot of energy is needed to break the bonds so they have low boiling and melting points.
Between phosphorus \left(\text{P}\right) and sulfur \left(\text{S}\right), there is a slight increase in melting/boiling point because the sulfur molecule \left(\text{S}_8\right) has more electrons than the phosphorus molecule \left(\text{P}_4\right), so it has stronger Van der Waals forces.
Argon \left(\text{Ar}\right) is a monatomic element (it exists as one atom) and therefore has weak Van der Waals forces and so a low melting/boiling point.
KeyNote: The trends in Period 3 are similar in Period 2, except that while lithium \left(\text{Li}\right) and beryllium \left(\text{Be}\right) are both metals, boron is not a metal and is in fact a macromolecule so will have a very high melting point compared to the metals.
Periodicity Example Questions
Question 1: Which block in the periodic table contains silver?
[1 mark]
The d-block.
Question 2: In terms of structure and bonding, explain why phosphorus has a lower melting point than sulfur.
[3 marks]
- Phosphorous \left(\text{P}_4\right) molecules are smaller and have more electrons than sulfur \left(\text{S}_8\right) molecules.
- So the Van der Waals forces between the molecules are weaker.
- So less energy is needed to overcome the forces between the molecules.
Question 3: Predict whether an atom of ^{137}\text{Ba} will have an atomic radius that is larger than, smaller than or the same as the atomic radius of ^{132}\text{Cs}. Explain your answer.
[3 marks]
- An atom of ^{137}\text{Ba} will be smaller.
- There will be a bigger nuclear charge in ^{137}\text{Ba}.
- But both ^{137}\text{Ba} and ^{132}\text{Cs} will have the same shielding.
Question 4: Give the electronic configuration of chlorine and explain why some elements in the periodic table are classified into the p-block.
[2 marks]
\text{Cl: }1\text{s}^22\text{s}^22\text{p}^63\text{s}^23\text{p}^5
Elements in the p-block have their outer electrons in a p orbital.