Fundamental Particles & Electronic Configuration
Fundamental Particles & Electronic Configuration Revision
Fundamental Particles & Electronic Configuration
Atoms are made up of three fundamental particles called protons, neutrons, and electrons which can determine the physical and chemical properties of elements. Electronic configurations are used to show the arrangement of electrons in an atom.
Protons, Neutrons and Electrons
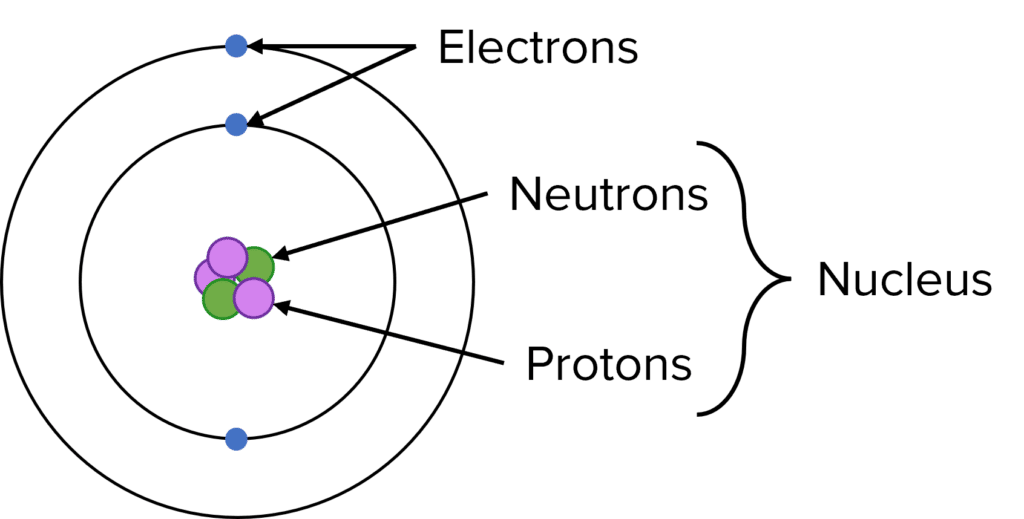

Protons and neutrons are fundamental particles that are found within the nucleus of an atom while electrons are found in orbitals around the nucleus.
Each fundamental particle has a relative mass and a relative charge.


Protons have a relative mass of 1 and a relative charge \text{+}1.
Neutron have a relative mass of 1 and a relative charge 0.
Electrons have a relative mass of \approx\dfrac{1}{1840} and a relative charge \text{-}1.
Mass Numbers and Atomic Numbers
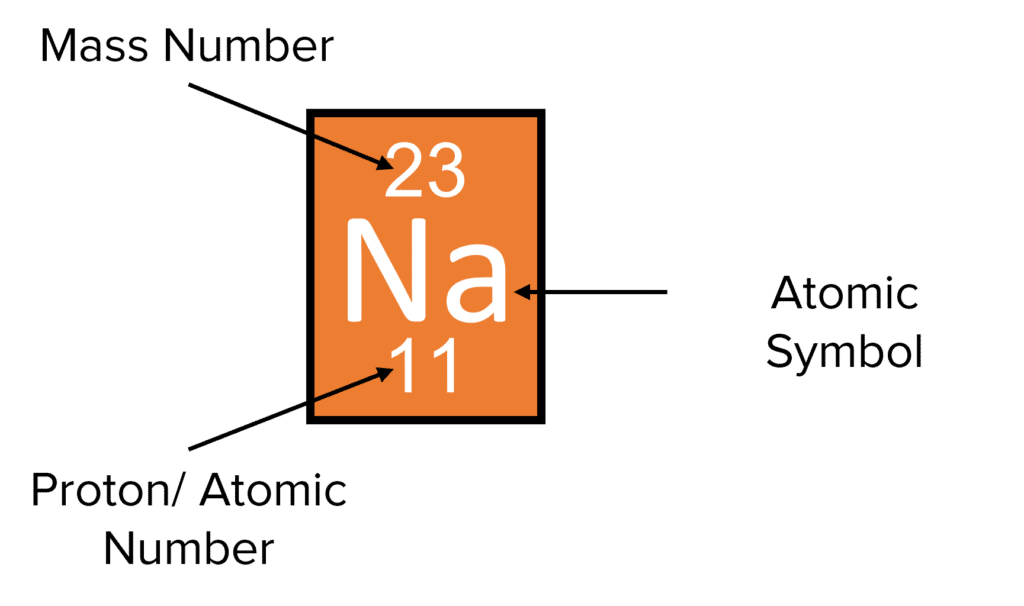

Atomic and mass numbers give important information about elements in the periodic table.
Atomic Number (Z) = Number of Protons. It is usually the smaller number associated with the element on the periodic table.
Mass Number (A) = Total Number of Protons + Neutrons. It is usually the bigger number associated with the element on the periodic table.
To work out the number of neutrons, the atomic number is subtracted from the mass number.
Example: Using the periodic table, work out the number of electrons in Fluorine (\text{F}).
\begin{aligned}\text{Atomic Number}&=9\\ \text{Mass Number} &= 19\\\text{Number of Neutrons} &= \text{Mass Number}-\text{Atomic Number}\\\text{Number of Neutrons} &= 19-9\\ &=10\end{aligned}
Isotopes
An isotope of an element is an atom with the same number of protons but a different number of neutrons.
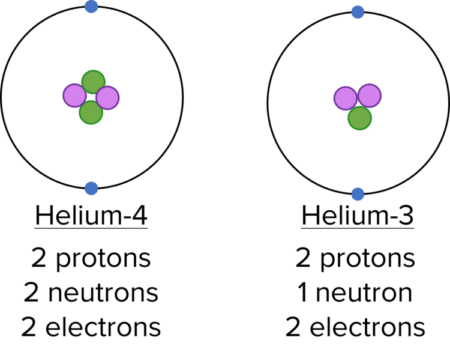
Isotopes of an element have the same electronic configuration, and therefore have similar chemical properties. However, their physical properties may vary due to the difference in mass.
Note: The names Helium-4 and Helium-3 are based on the mass number of the atom.
Electronic Arrangement
Electrons are arranged into principle energy levels, sub-energy levels and orbitals.
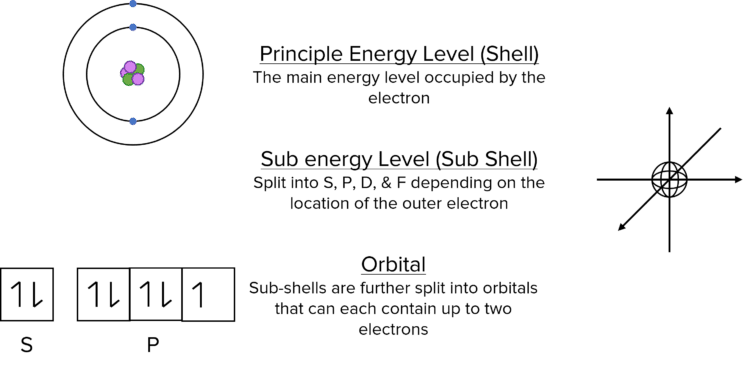
Principle Energy Levels
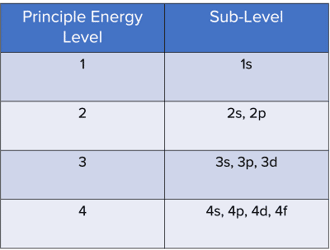

Principle energy levels increase in energy as you move away from the nucleus, so principle energy level 2 is higher in energy than principle energy level 1 and principle energy level 3 is higher in energy than principle energy level 2. Each principle energy level can be divided into sublevels.
Sub-Energy Levels
The periodic table is divided into different blocks. The block an element is in will depend on the subshell that the outermost electrons are in. The four blocks of the periodic table are the s, p, d and f blocks.
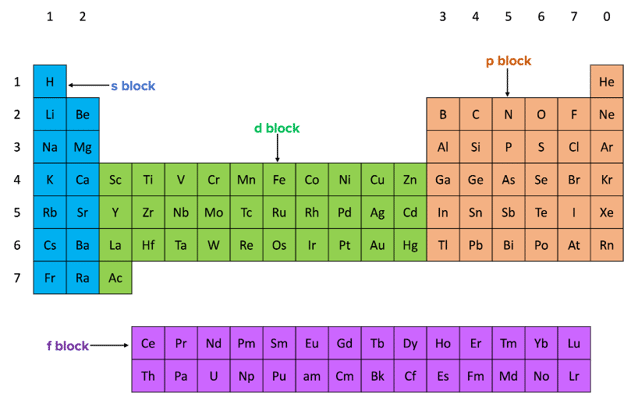
- Elements in the s block have their outer electrons in an s subshell. These subshells contain one spherical s orbital that can hold up to 2 electrons.
- Elements in the p block have their outer electrons in a p subshell. These subshells can hold up to 6 electrons and contain 3 p orbitals.
- Elements in the d block have their outer electrons in a d subshell. These d subshells can hold up to 10 electrons and contain 5 d orbitals.
- Elements in the f block have their outer electrons in an f subshell. These f subshells can hold up to 14 electrons and contain 7 f orbitals.
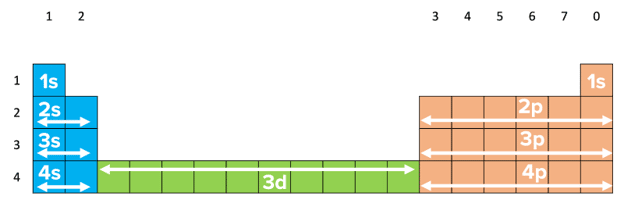
The s subshell has the lowest energy followed by p, and then d. The f sub-shell has the highest energy. Electrons fill subshells from the lowest energy to the highest energy and we use electronic configurations to show this.
Example: What are the electron configurations for Fluorine (\text{F}) and Magnesium (\text{Mg})?
Fluorine (\text{F}) = 1\text{s}^2 2\text{s}^2 2\text{p}^5
Magnesium (\text{Mg}) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2
Note: The 4s subshell fills before the 3d subshell because the 4s subshell is lower in energy than the 3d subshell. So the electronic configuration for titanium would be: 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 4\text{s}^2 3\text{d}^2 .
Ions and d-block Elements
When an ion is formed, an atom either gains electrons in its outer shell or loses electrons from its outer shell.
Example: What is the electron configuration for a sodium ion \left(\text{Na}^+\right)?
Sodium (\text{Na}) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^1
Sodium Ion (\left(\text{Na}^+\right) = 1\text{s}^2 2\text{s}^2 2\text{p}^6
When elements in the d-block form positive ions, the electrons are removed from the 4s subshell before the 3d subshell.
Example: What is the electron configuration for a Nickel ion \left(\text{Ni}^{2+}\right)?
Nickel (\text{Ni}) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 4\text{s}^2 3\text{d}^8
Nickel Ion (\left(\text{Ni}^{2+}\right) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 3\text{d}^8
Electron configurations can also be written in a shortened form to avoid really long configurations. To do this, find the noble gas from the previous period, this represents the electronic configuration up to that point, and then write out the rest of the configuration.
Example: What is the shortened electron configuration for manganese?
Manganese (\text{Mn}) = [\text{Ar}] 4\text{s}^2 3\text{d}^5
Exception
Electrons favour half or fully filled d subshells because of the symmetrical distribution of electrons.
Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d subshell. For chromium, the 3d subshell is made to be half full leaving the 4s subshell with only one electron. Similarly, for copper, the 4s subshell is left with only one electron to allow for the 3d subshell to be completely full.
Electron Spin Diagrams
Sublevels are made up of orbitals that can hold up to 2 electrons. Spin diagrams are used to represent these orbitals. The following is an example of an electron spin diagram.
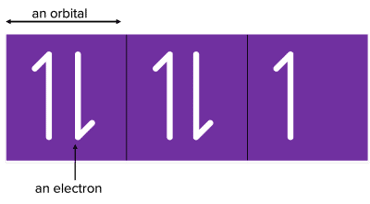

Key points:
- We draw a half arrow to represent an electron.
- The electrons are in opposite directions to show the different spins of the electrons in the orbital. Different spins are what allow the two negatively charged electrons to remain in the same orbital without significant repulsion.
- When filling up subshells, fill in each orbital with one electron before beginning to pair them up. This is because electrons first enter the orbitals that are empty before filling singularly occupied orbitals.
Example: Draw the electron spin diagram for a nitrogen atom.

Example: Electron configurations
What are the electron configurations for an oxygen ion \left(\text{O}^{2-}\right) and a Vanadium Ion \left(V^{3+}\right)?
[2 marks]
Oxygen (\text{O}) = 1\text{s}^2 2\text{s}^2 2\text{p}^4
Oxygen Ion \left(\text{O}^{2-}\right) = 1\text{s}^2 2\text{s}^2 2\text{p}^6
Vanadium (\text{V}) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 4\text{s}^2 3\text{d}^3
Vanadium Ion \left(\text{V}^{3+}\right) = 1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 3\text{d}^2
Fundamental Particles & Electronic Configuration Example Questions
Question 1: What is the electron configuration for calcium (\text{Ca})?
[1 mark]
1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6 4\text{s}^2
or
[\text{Ar}] 4\text{s}^2
Question 2: What is the electron configuration for calcium ion \left(\text{Ca}^{2+}\right)?
[1 mark]
1\text{s}^2 2\text{s}^2 2\text{p}^6 3\text{s}^2 3\text{p}^6
Question 3: Which fundamental particles would be deflected by an electric field?
[3 marks]
Protons and electrons as they posses an electric charge.
Question 4: In terms of fundamental particles, state the difference between isotopes of an element and why they have the same chemical properties.
[2 marks]
Different number of neutrons. Same number of protons
Same electron configuration.
Question 5: Draw the electron spin diagram for a fluorine atom.
[2 marks]

One mark for the correct number of orbitals for each sub-level.
One mark for the correct number of electrons in each sub-level and correct orientation.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.