Ionisation Energies
Ionisation Energies Revision
Ionisation Energies
Ionization energy is the energy require to remove one electron from an atom or ion, increasing its charge. It is defined as the enthalpy change when one mole gaseous atoms form gaseous ions with positive charges. There are a number of factors that will affect the ionisation energy of an atom (or ion), as well as trends in these energies both within atoms and across periods.
First and Second Ionisation Energy
First Ionisation Energy: The enthalpy change when one mole of gaseous atoms forms one mole of gaseous ions with a single positive charge.
Half Equation: \text{K(g)} \rarr \text{K}^+ \text{(g)} + \text{e}^-
Second Ionisation Energy: The enthalpy change when one mole of gaseous ions with a single positive charge forms one mole of gaseous ions with a double positive charge.
Half Equation: \text{Cu}^+\text{(g)} \rarr \text{Cu}^{2+} \text{(g)} + \text{e}^-
Note: The equations for ionisation energy are always the same regardless of the usual ions the element forms or usual states the element is in.
Factors Affecting Ionisation Energy
There are three factors that primarily affect ionisation energy:
- Nuclear charge – the more protons in the nucleus the greater the nuclear charge and the greater the attraction of the electrons to the nucleus.
- Size of the atom – The outer shell of bigger atoms will be further from the nucleus so there would be a weaker attraction between the nucleus and the electrons.
- Shielding – The more shells there are the weaker the attraction of the outer shell to the nucleus.
Successive Ionisation Energies
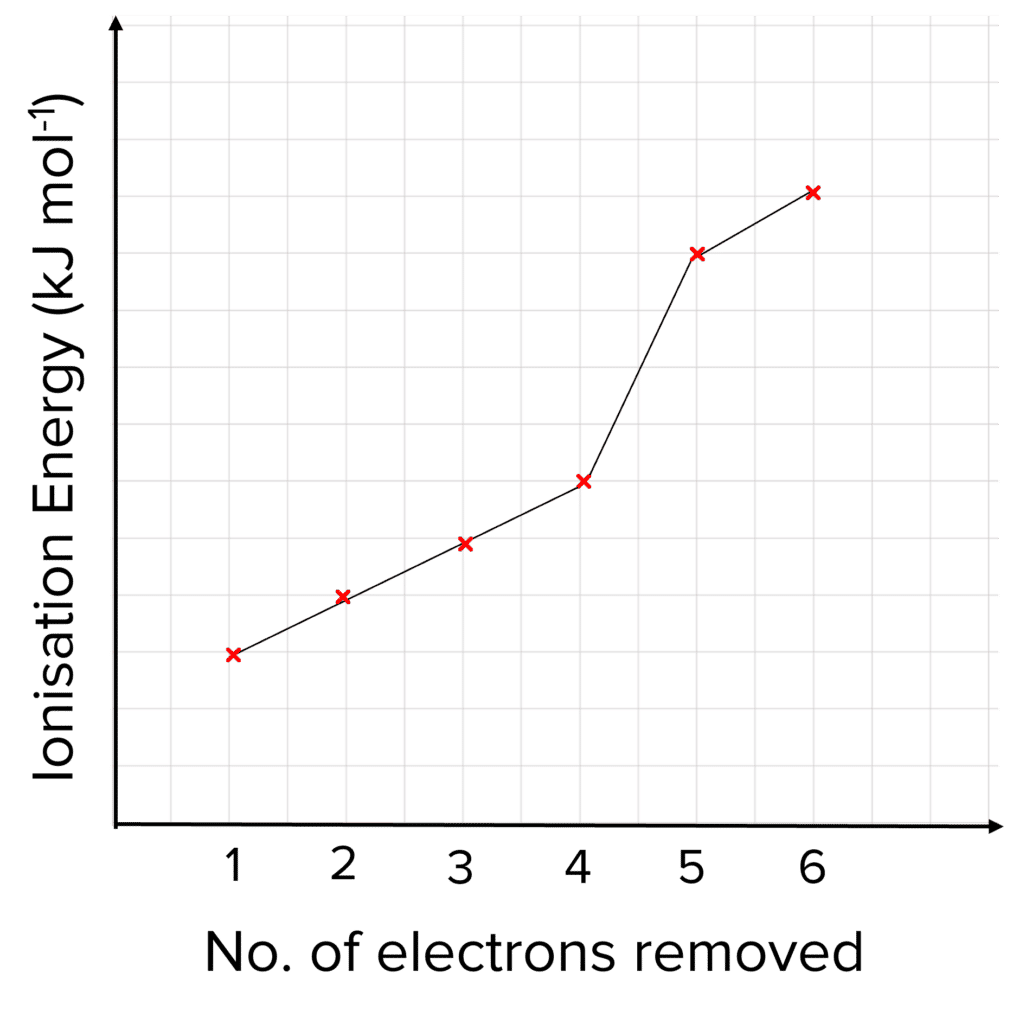

Through looking at patterns in successive ionisation energies, we can deduce key information about the electronic structure of an element.
We can use a graph of ionisation energies to tease out information about unknown elements. For example, by looking for jumps in ionisation energies, the group that this element belongs can be determined.
The large jump in the graph to the right shows shows the successive ionisation energies of a group 4 element. We see that the 5th electron must be in a different shell to the other electrons, as it experiences a much greater attraction to the positively charged nucleus. More energy is required to remove the electron due to the stronger attraction. If there are 4 electrons in the outer shell, that tells us that the element must be a group 4 element.
The first ionisation energy is always lower than the second. This is because each time an electron is removed, the attraction between the outer electrons and nucleus becomes stronger, making it harder to remove the next electron so more energy is required.
Patterns in First Ionisation Energy
There are a few patterns in ionisation energies across a period that can increase our understanding of electronic structures.
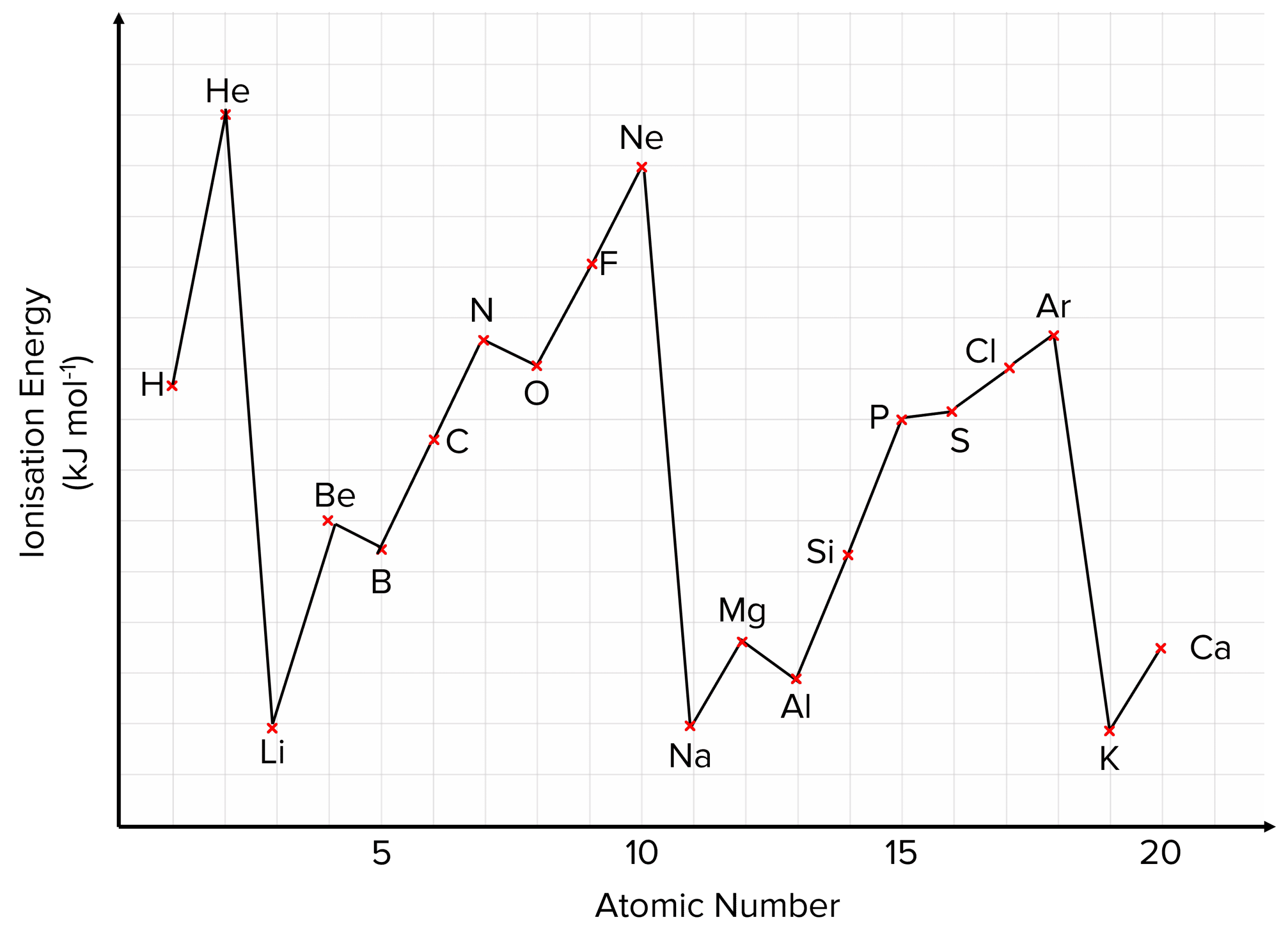
- Helium has the highest first ionisation energy. The first electron removed in helium has no shielding effect and is very close to the nucleus so it requires a lot of energy to remove. The ionisation energy is higher than hydrogen because it has more protons.
- First ionisation energy decreases down a group. As you go down a group, there is an additional shell for each element so the outer electrons are further from the nucleus and have a weaker attraction to it. This means that less energy is required to remove the first electron. This is shown in the graph by its general downward slant.
- Electrons are removed more easily from orbitals containing two electrons due to electron-electron repulsion. There are also changes in first ionisation energy across a period. This can be seen in the spikes in the graph for Helium, Beryllium, Neon, Magnesium and Argon. All of these elements contain their outer electrons in doubly occupied orbitals Patterns across a period are referred to as periodicity.
- First ionisation energy generally increases across a period. The number of protons in the nucleus increases across a period, while the shielding remains the same. There is therefore an increasing attraction of the outer electrons to the nucleus which makes it harder for an electron to be removed from the outer shell. We can see this in the increase in ionisation energies between lithium and neon.
Exceptions:
Magnesium and Aluminium– between Magnesium (\text{Mg}) and Aluminium (\text{Al}), there is a drop in the 1st ionisation energy. This happens because:
- The outer electron of \text{Mg} is in the 3s subshell while the outer electron of \text{Al} is in the 3p subshell.
- So when removing an electron from the outer shell of (\text{Al}), the electron is being removed from a 3p subshell which has a higher energy than the 3s subshell.
- The higher energy level makes it easier for an electron to be removed, decreasing the energy required to remove the electron.
Phosphorous and Sulfur– there is also a drop in 1st ionisation energy, between Phosphorus (\text{P}) and Sulfur (\text{S}). This happens because:
- Although the outer electrons in both phosphorous and sulfur are in 3p orbitals, the outer electron of sulfur is paired up with another electron while the outer electron of phosphorous is not.
- This causes a small repulsion between the two electrons making it easier for an electron to be removed.
Note: For the graph of the second ionisation energy of elements, the graph is shifted one to the left.
Ionisation Energies Example Questions
Question 1: Give the definition of second ionisation energy and write the half equation for the second ionisation of magnesium.
[3 marks]
The enthalpy change when one mole of gaseous ions with a single positive charge forms one mole of gaseous ions with a double positive charge.
\text{Mg}^+\text{(g) ---> Mg}^{2+}\text{(g) + e}^{-}
Question 2: Explain why the first ionisation energy of neon is higher than the first ionisation energy of sodium.
[2 marks]
- Outer electron in neon is removed from a shell of lower energy OR smaller atom OR electron nearer to the nucleus.
- Electron is removed from 2p rather than from 3s subshell.
Question 3: State how, and explain why, the values of the first ionisation energies of the elements aluminium (\text{Al}) and sulfur (\text{S}) deviate from the general trend.
[4 marks]
- For aluminium: (third) electron in (3) p sub-shell.
- Sub-shell further away from nucleus OR of higher energy OR extra shielding.
- For sulphur: Pair of electrons in (3) p orbital.
- Repulsion between electrons.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.