Electronegativity & Intermolecular Forces
Electronegativity & Intermolecular Forces Revision
Electronegativity & Intermolecular Forces
Electronegativity is, in essence, how strongly an atom will pull a pair of electrons towards itself in a covalent bond. Electronegativity will have a significant impact on the behaviour of covalent molecules by affecting the polarity of the overall molecule. Bonds between atoms of significantly different electronegativities will be significantly more polar than those between atoms of similar electronegativities. Electronegativity can be measured using the Pauling scale.
Trends in, and Factors affecting, Electronegativity
Electronegativity increases as we move towards the top right hand corner of the periodic table. This trend does not include the Nobel gasses however. This is because electronegativity only has an effect in covalent bonds. As the Nobel gasses do not form molecules, they can not display any electronegativity. The Pauling Scale measures electronegativity and ranges from 0 - 4, with 4 being the most electronegative, 0 the least.
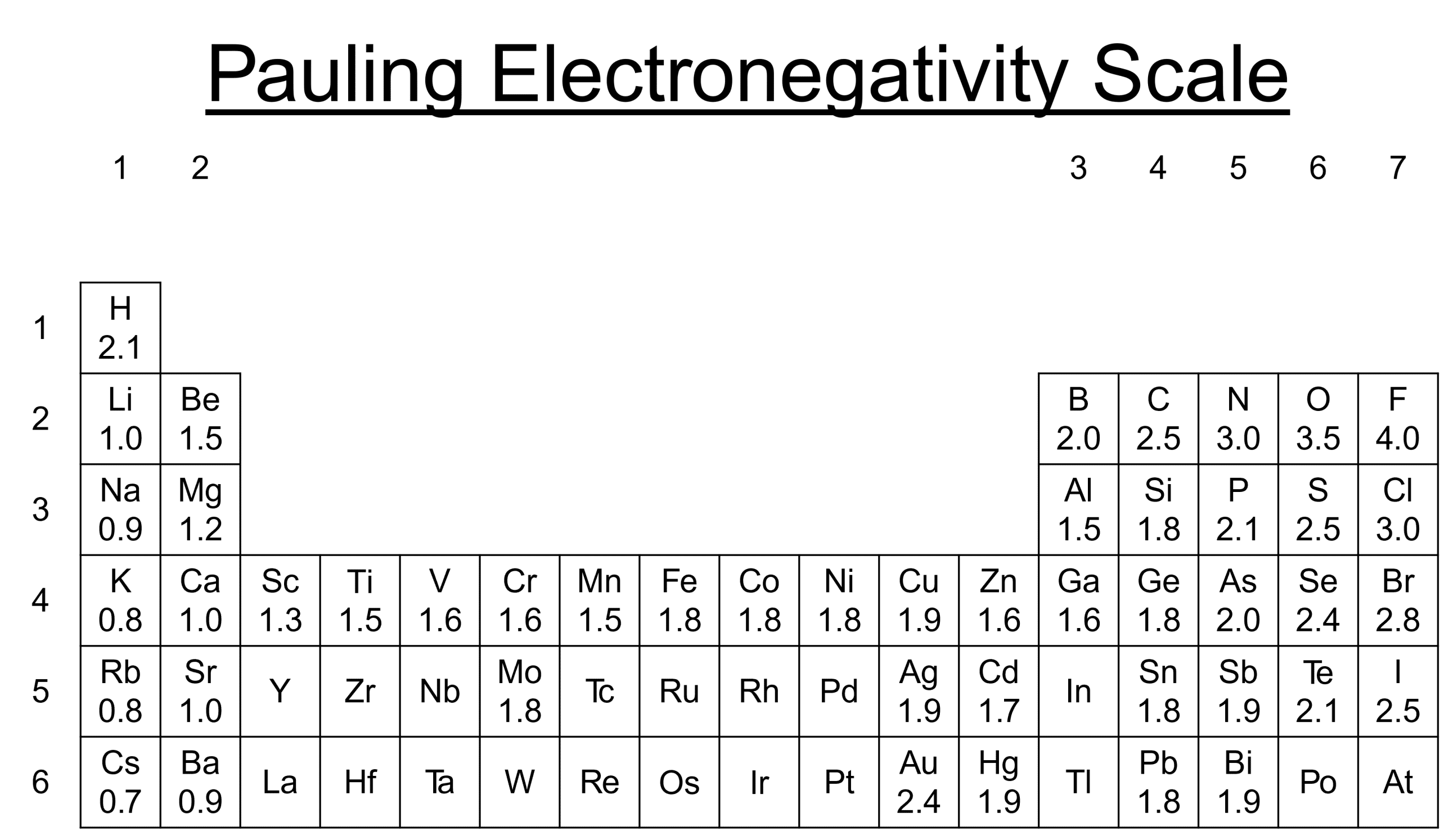
The most electronegative element is fluorine \text{(F)} which has an electronegativity of 4.0. Fluorine, Oxygen \text{(O)}, Nitrogen \text{(N)} and Chlorine \text{(Cl)} are the most electronegative atoms.
Electronegativity decreases down a group because there is an increase in the distance between the nucleus and outer electrons. In addition to this, the shielding also increases due to more (inner) shells. Electronegativity increases across a period because the nuclear charge increases which means outer electrons have a stronger attraction to the nucleus.
Bond Dipoles
The most important result of electronegativity its the creation of polar bonds. If electronegativity was not a factor then the electrons in a covalent bond would be shared perfectly in the centre of the bond. In reality, the differences in electronegativity with in bonds will shift the electron pair towards which ever atom is the most electronegative.
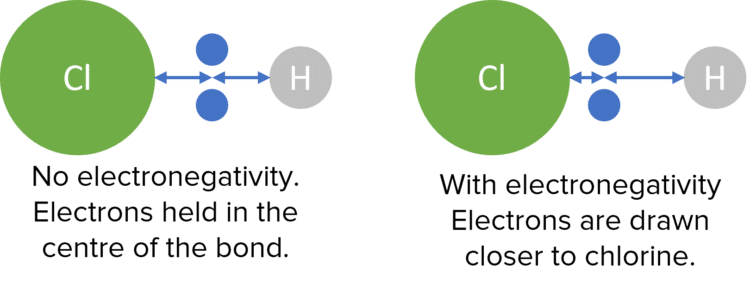
This will pull electron density towards one side of the bond, creating an area of high density and one of low density. The concentration of electron density at one end of the bond will lead to a slight negative charge (called a partial charge) at this end, and a slight positive charge at the end with a low concentration of electron density. We represent these partial charges with a lower case Greek delta . This difference in charge is known as a dipole.
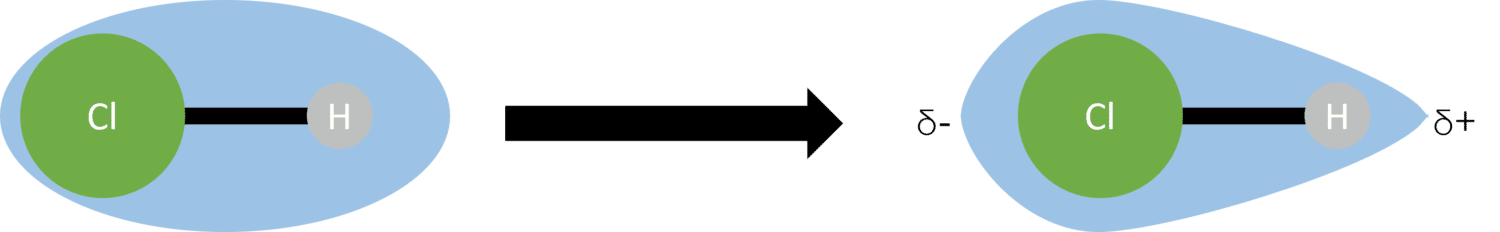
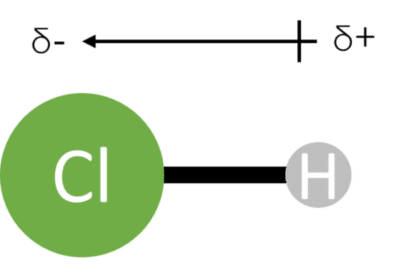
To represent a dipole, a special arrow can be drawn. The arrow is drawn from the positive (\delta +) side of the bond to negative (\delta -) end, with a plus at the beginning:

Molecular Dipoles
Covalent molecules will often contain many bond dipoles, often pointing in different directions. The directions of these dipoles will influence the overall dipole of the molecule. A number of factors will affect how an overall dipole is formed, including the shape of the molecule and its constituent atoms.

Diatomic molecules (molecules consisting of only two atoms) will contain only one dipole as they contain only one bond. When the molecule contains only atoms of the same elements – for example \text{H}_2 or \text{Cl}_2 – there will be no overall dipole as there will be no difference in electronegativity across the bond.
If the atoms in the molecule are of different elements – for example \text{HCl} – there will be an overall dipole. This overall dipole will be in the same direction as the bond dipole.
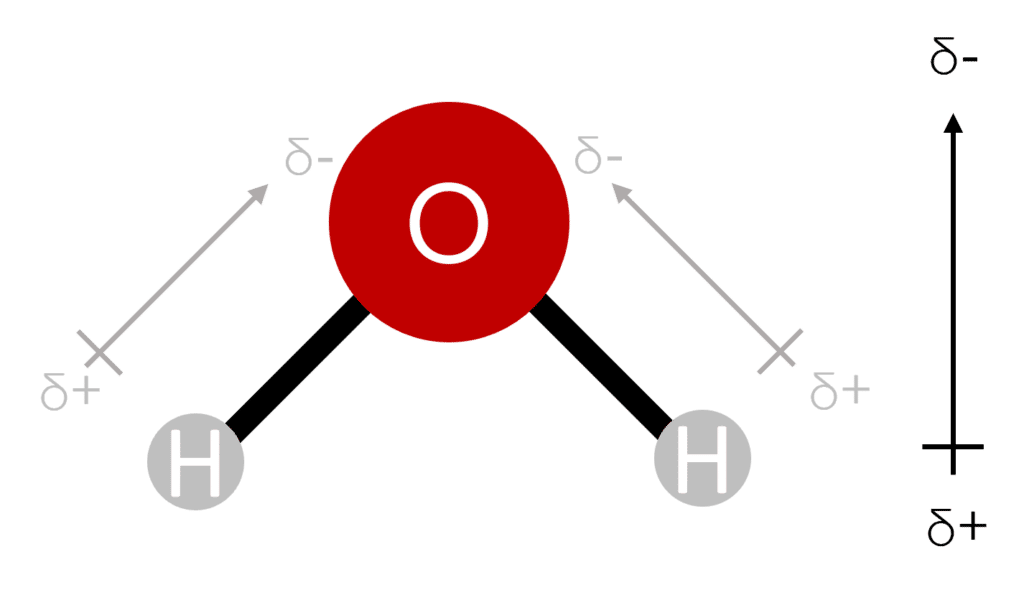

For molecules containing more than two atoms (polyatomic) of different elements, the presence of dipoles will depend on the shape of the molecule. If a molecule contains multiple bond dipoles with different orientations, then there will only be an overall dipole if more than half of the bond dipoles point in roughly the same direction. This is the case in the example of water (\text{H}_2\text{O}. Here we have two bond dipoles that both point towards the central oxygen atom.
Because \text{H}_2\text{O} has a bent shape, the bond dipoles also point slightly “upwards”. This creates the overall dipole of the water molecule.
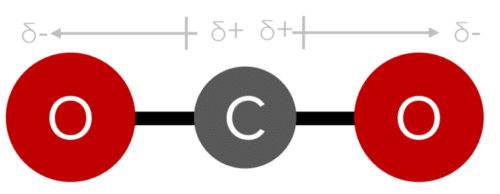
Without the bent shape, water would not display an overall dipole. In a linear and symmetrical molecule, the bond dipoles will cancel each other out. This is the case in \text{CO}_2. Here we have two bond dipoles of equal magnitude, oriented in opposite directions away from the central carbon atom. Because \text{CO}_2 is linear, these dipoles are directly opposed, unlike in water where the bent shape creates an angle between the bond dipoles. it is this direct opposition that prevents an overall dipole from forming.
Van der Waals’ Forces
Van der Waals’ forces are a type of intermolecular force that happens between noble gases and other molecules.
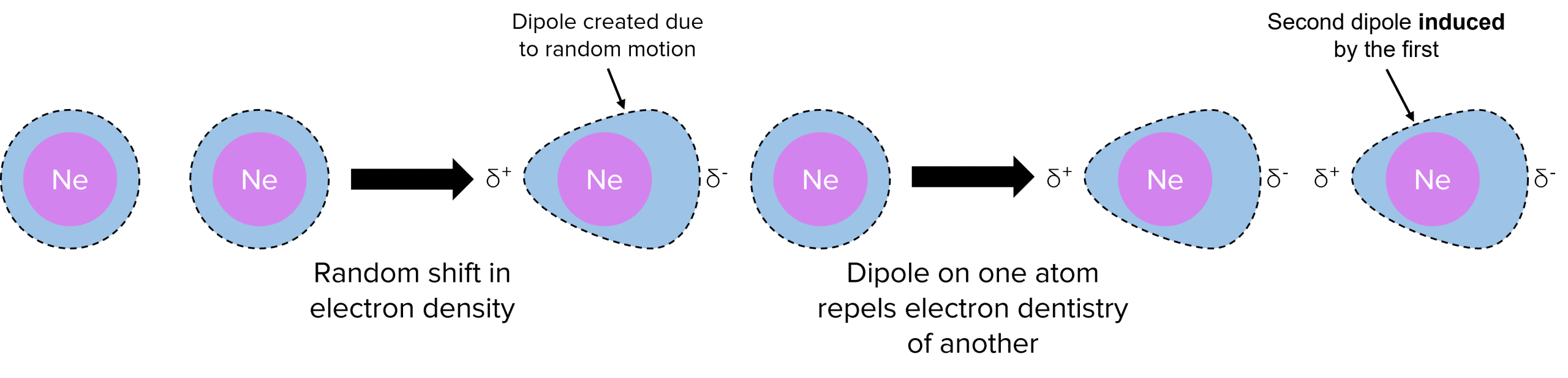
Electrons are constantly moving around randomly so electron density can shift causing some parts of a molecule to become more negative than other parts.
The transient (temporary) dipoles formed can cause molecules nearby to form dipoles as well. These are known as induced dipoles. These induced dipoles lead to electrostatic attraction between molecules.
- Van der Waals’ forces do not occur in ionic substances.
- The stronger the van der Waals forces between molecules, the higher the boiling point of the molecules.
Factors affecting van der Waals’
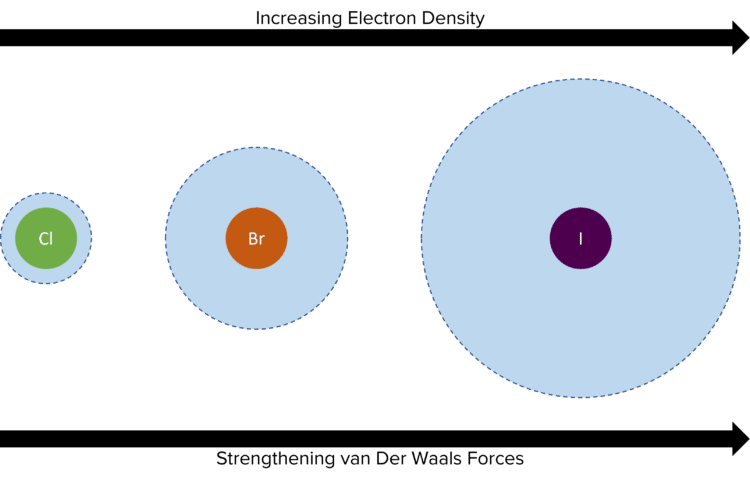
The number of electrons is the main factor that affects the strength of van der Waals’ forces.
- The more electrons there are in a molecule, the stronger the van der Waals’ forces will be and therefore, the higher the boiling point will be.
- More electrons means it is more likely for induced dipoles to form.
The shape of a molecule can also affect the strength of van der waals’ forces
- Branched isomers of an alkane have a smaller surface area which means that weaker Van der Waals’ forces are formed.
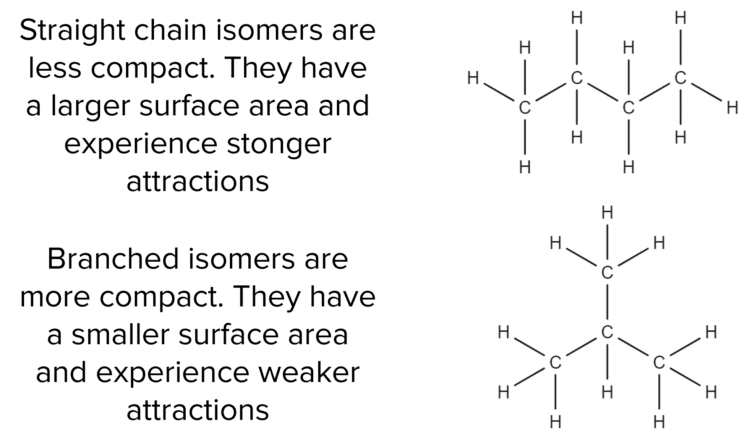
- The presence of chain branching or of side groups (such as chlorine or alcohols) in an alkane will prevent the chains from getting close together. This reduces the strength of the Van der Waal’s forces between them.
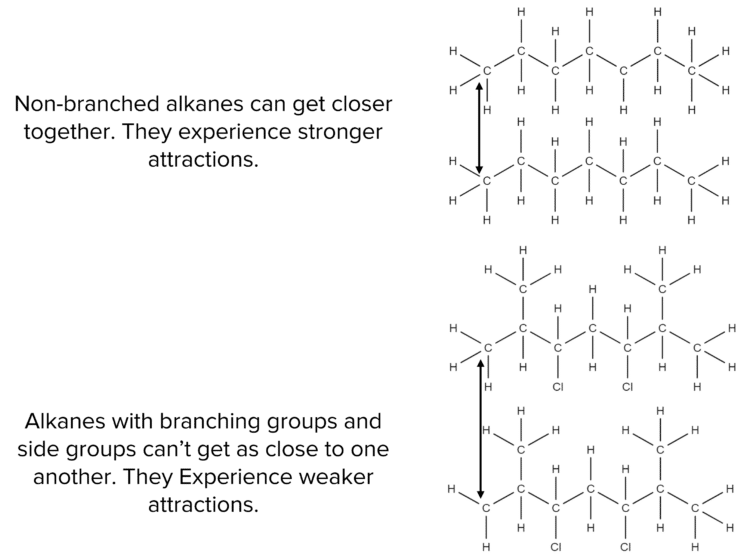
- Long chain alkanes have a larger surface area so can form stronger of van der Waals’ forces since there are more points of contact between molecules.
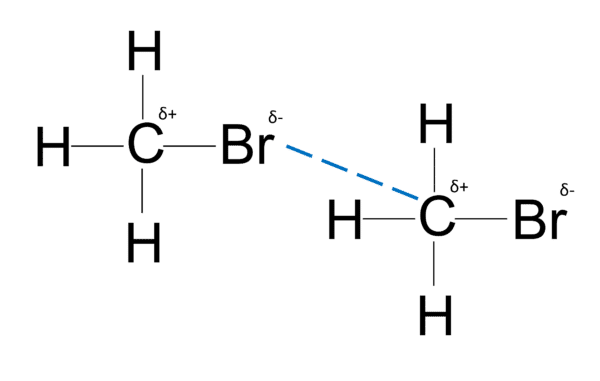
Permanent dipole-dipole Forces
- Dipole-dipole forces form between polar molecules which have a permanent dipole. \text{CO}, \text{HBr}, \text{CF}, \text{CCl} and \text{CBr} are the most common bonds that can form permanent dipoles.
- Permanent dipoles can occur when there is a large difference in electronegativity between bonded atoms.
- Dipole-dipole interactions are stronger than van der waals’ forces.
- Compounds with permanent dipole-dipole forces usually have higher boiling points than compounds with just Van der Waals’ forces.

Hydrogen Bonding
Hydrogen bonding is the strongest type of intermolecular bonding
Hydrogen bonding happens in compounds where a hydrogen atom is attached to one of the three most electronegative atoms in the Pauling scale; fluorine, oxygen, and nitrogen. Hydrogen bonding occurs because of the large difference in electronegativity between hydrogen atoms and fluorine, oxygen or nitrogen atoms. Amides, alcohols, proteins, carboxylic acids, and water can all form hydrogen bonds since they contain hydrogen atoms bonded to at least one atom of the three most electronegative elements.
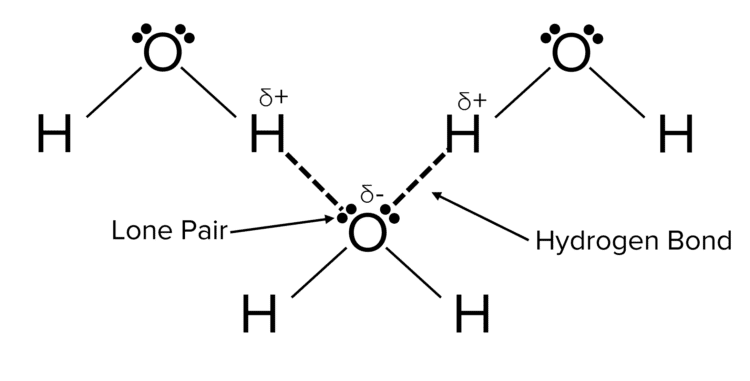
It is very likely that you will be asked to draw diagrams that show hydrogen bonding. It is essential to draw the lone pairs and partial charges in these diagrams. We represent the hydrogen bond by drawing a dotted line from the lone pair of the \delta^- atom to the \delta^+ hyrdogen.
Note: Molecules can experience hydrogen bonding, permanent dipole-dipole forces and van der waals’ forces simultaneously.
Electronegativity & Intermolecular Forces Example Questions
Question 1: State the meaning of electronegativity.
[2 marks]
- The ability (/ power/ tendency) of an atom to withdraw (/ attract electron density / electron cloud/ electron pair to itself.
- In a covalent bond.
Question 2: Explain why \text{HCl} has a lower boiling point than \text{HF}. Give your explanation in terms of intermolecular forces.
[3 marks]
- There is hydrogen bonding in \text{HF}.
- There is permanent dipole-dipole bonding/ van der waals’ in \text{HCl}.
- Hydrogen bonding is stronger/ hydrogen bonding is the strongest intermolecular force.
Question 3: Propanone has the following structure:
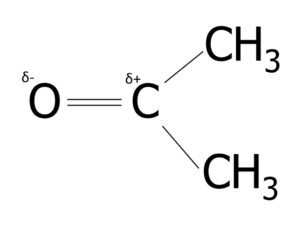
Explain why the \text{C=O} bond in a propanone molecule is polar.
[2 marks]
- Oxygen is more electronegative than carbon.
- Which causes higher electron density around the oxygen atom.
Question 4: Explain why the Van der Waals’ forces in liquid argon are very weak. Give your explanation in terms of atomic structure.
[2 marks]
- Argon is a single atom with electrons close to the nucleus.
- This makes it harder to polarise it/ distort the electron cloud.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.