Bonding and Structure
Bonding and Structure Revision
Bonding and Structure
Bonding is one of, if not the most important phenomena in chemistry. The bonding between atoms or ions in substances will control both those substances chemical properties as well as their structural and physical properties. The three main bonding types are ionic, covalent, and metallic. All three types of bonding can be described in terms of the distribution of electrons within the molecules.
Ionic Bonding
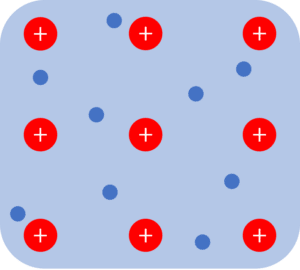
An ionic bond happens when a non-metal and metal react together. When an ionic bond forms, the metal atom loses electrons while the non-metal atom gaining electrons. This causes the metal to become positively charged while the non-metal atom become negatively charged. The formation of two oppositely charged ions leads to the formation of a strong electrostatic attraction between them.
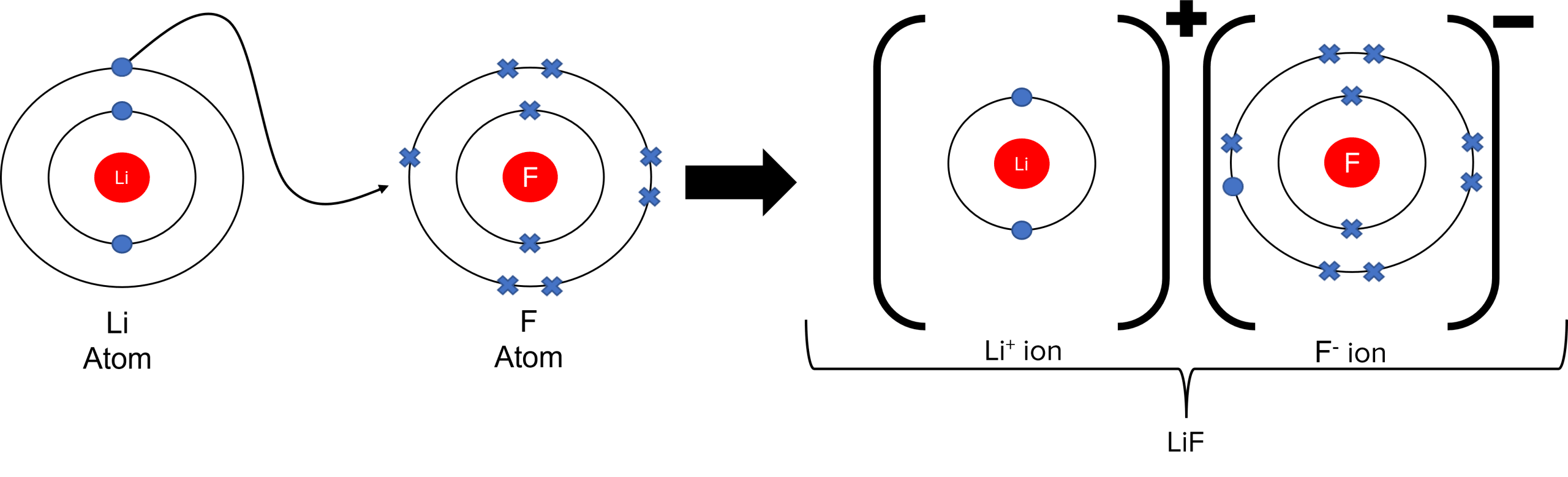
Smaller ions or ions with higher charges can form stronger ionic bonds between atoms which leads to higher melting points. Ionic substances are generally giant ionic lattices. These lattices will typically have high melting points, be soluble in water, and conduct electricity when molten or dissolved in solution.
Covalent and Dative Covalent Bonding
Covalent bonding occurs when atoms share outer pairs of electrons.
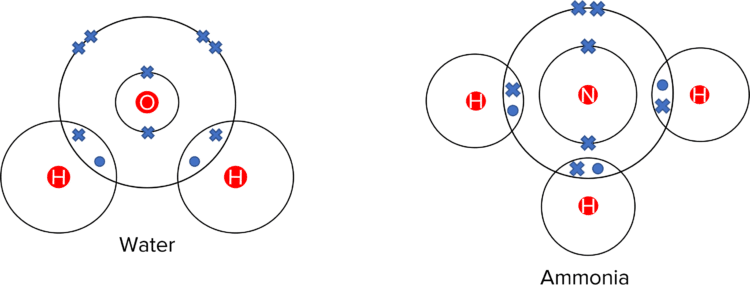
Dative covalent bonding, also known as coordinate bonding, occurs when one of the bonding atoms in a covalent bond donates a pair of electrons. This is shown by an arrow going from the atom that donates an electron pair to the atom that accepts the electron pair.
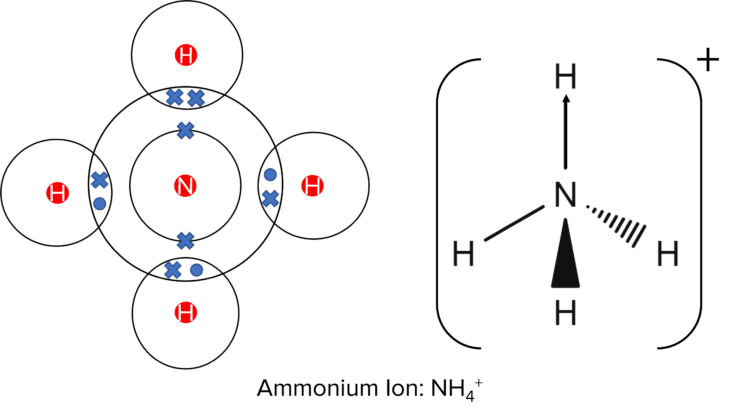
Covalent bonding happens in simple molecular and macromolecular substances. Simple molecular substances, such as \text{H}_2\text{O} and \text{NH}_3, are often gases and liquids. Macromolecules, such as \text{SiO}_2, are solid. They can also be called giant covalent structures. Simple molecules usually have low melting and boiling points while macromolecules have higher boiling points because of their many covalent bonds.
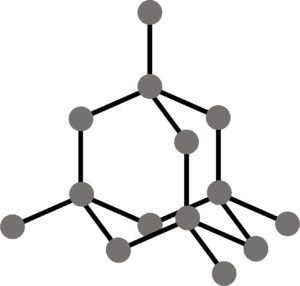
Metallic Bonding
Metallic bonding involves electrostatic forces of attraction between a lattice of positively charged metal ions and a sea of delocalised electrons.
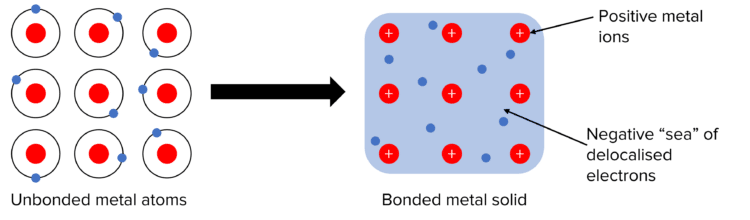
Factors Affecting Metallic Bonding
- Nuclear attraction – More protons in the nucleus mean that there is a higher nuclear charge and therefore more attraction of the electrons to the nucleus and a stronger bond.
- The number of delocalised electrons – More delocalised electrons will lead to greater forces of attraction between the sea of electrons and the metal ions. This leads to stronger bonds.
- Size of the ion – A smaller ion will form stronger bonds because of its higher charge density.
In terms of physical properties, metals are usually shiny. They are also ductile and malleable since the layers of metal ions in the lattice can slide over each other. Metals also have high melting and boiling points because of the strong forces of electrostatic attraction.
Structures of Solids
| Ionic | Metallic | Simple Molecular | Macromolecular | |
| Structure | 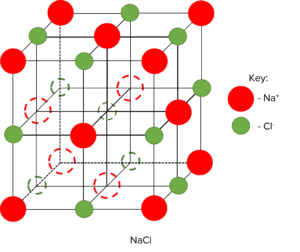 |
|
 |
|
| Melting and boiling point | High due to giant lattice | High due to strong electrostatic forces of attraction | Low due to weak intermolecular forces between the molecules | High due to the many strong covalent bonds |
| Conductivity when solid | Low since ions in the lattice cannot move | Good since delocalised electrons can move | Low since no ions or delocalised electrons |
Generally poor since electrons cannot move (Graphite is an exception as it has delocalised electrons so it can conduct) |
| Conductivity when molten | High since ions are free to move and carry charge | High | Low since no ions or delocalised electrons | Low since no ions or delocalised electrons |
| Solubility in water | Good | Insoluble | Poor | Insoluble |
| Examples | Sodium Chloride (\text{NaCl}) Magnesium Oxide (\text{MgO}) |
All Metals | Iodine \left(\text{I}_2\right) Ice Water \left(\text{H}_2\text{O}\right) Methane Carbon Dioxide |
Graphite Diamond Silicon Silicon Dioxide |
Bonding Angles
Due to the (technically) unlimited nature of their size, it is not possible to talk about the distinct shapes of lattices, metal structures, or giant covalent compounds. Simple molecules are however small enough and limited enough to define their shapes. The shapes of simple molecules are determined by two things
1. The number of bonding pairs of electrons in the molecule
2. The number of lone pairs in the molecule.
Covalent bonds are formed by the sharing of two negative electrons between atoms. This means covalent bonds have a negative charge. As a result, the bonds in a molecule will experience electrostatic repulsions from other bonds, causing them to adopt arrangements that put them as far apart as possible. For example, a molecule with two chemical bonds and no lone electron pairs (such as \text{CO}_2) will place those bonds on either side of the central atom. The resulting molecule is said to be linear.
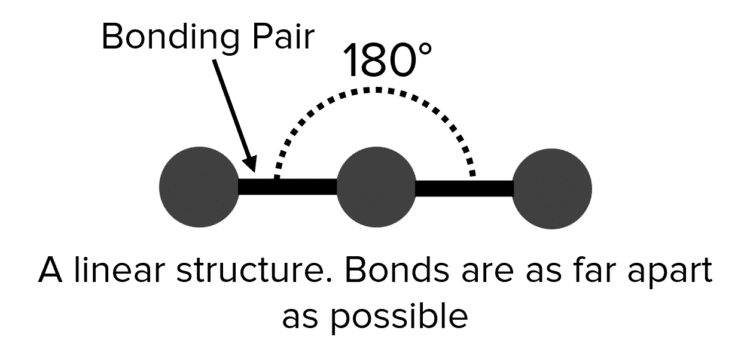
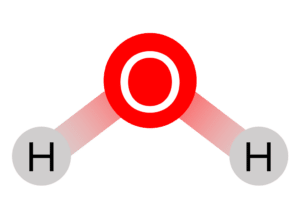
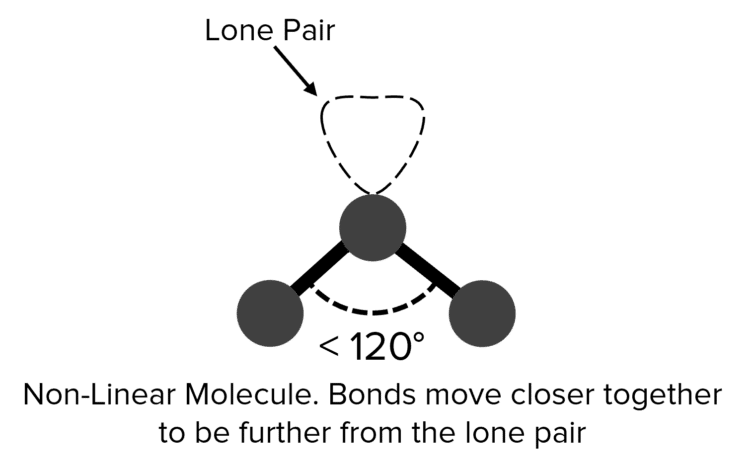
The presence of loan pairs in a molecule will complicate this picture. Lone pairs are held closer to the atom they belong to than a bonding pair. This greater proximity increases the electrostatic repulsion between the lone pairs and other bonding pairs. In this case, the bonding pairs will move closer together, trading off their repulsion of each other against the maximized distance from the lone pair. In the case of a molecule with two bonding pairs and one lone pair, this trade off will lead to the bonding pairs moving closer together, giving a bent or non-linear shape.
When representing molecules whos shapes are not flat (such as linear and bent molecules) we represent this 3d structure using solid and dashed wedges to represent bonds. For example, it is possible to represent the tetrahedral shape of methane in the following ways:
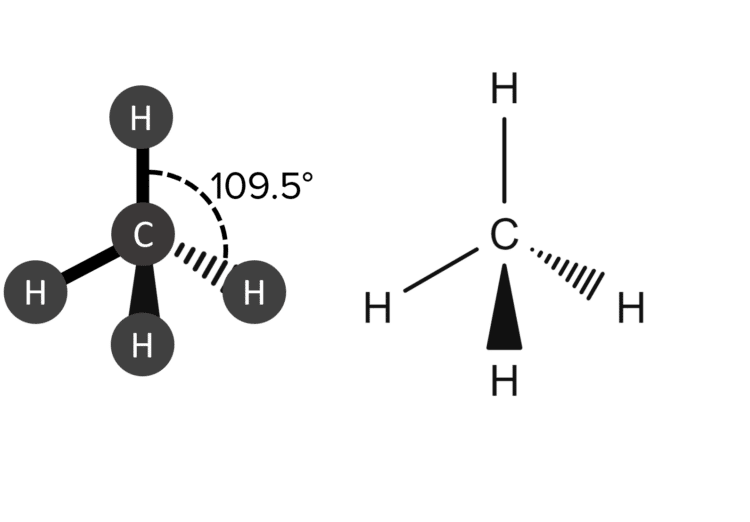
To represent bonds that are point forwards – that is out of the plane of the page – we used a solid wedge. To represent bonds that point backwards -that is into the plane of the page – we use a dashed wedge.
There are a number of different molecular shapes of varying complexity. The table below provides some examples.
This complex balance of proximities leads to a variety of unique molecular shapes. The table below summarises a few of these shapes, but is by no means exhaustive.
| Number of Bonding Pairs | Name | Structure | Bond Angles | Number of Lone Pairs | Examples |
| 2 | Linear | 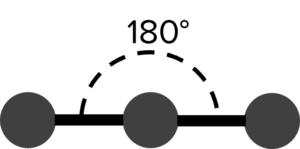 |
180\degree | 0 | Carbon Dioxide \left(\text{CO}_2\right) |
| Non-Linear | 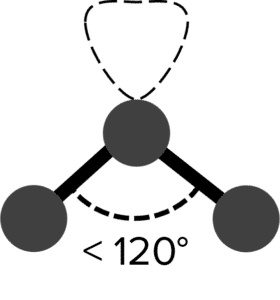 |
<120\degree | 1 | Tin Chloride \left(\text{SnCl}_2\right) | |
| Bent | 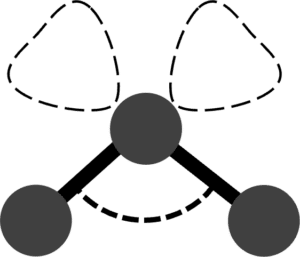 |
104.5\degree | 2 | Water \left(\text{H}_2\text{O}\right) | |
| 3 | Trigonal Planar | 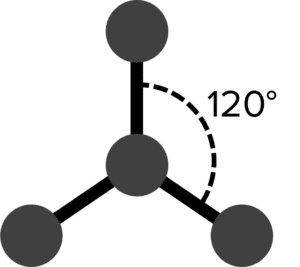 |
120\degree | 0 | Carbonate Ion \left(\text{CO}_3^{2-}\right) |
| Trigonal Pyramid | 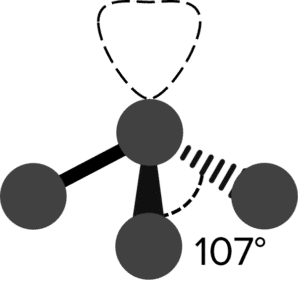 |
107\degree | 1 | Ammonia \left(\text{NH}_3\right) | |
| 4 | Tetrahedral | 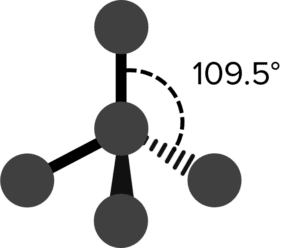 |
109.5\degree | 0 | Methane \left(\text{CH}_4\right) |
| Square Planar | 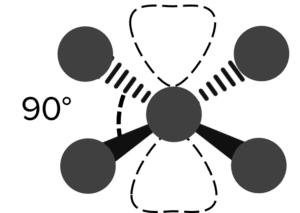 |
90\degree | 2 | Cisplatin \left(\text{PtCl}_4\right) | |
| 5 | Trigonal Bipyramid | 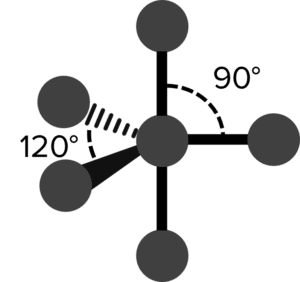 |
120\degree \text{ and } 90\degree | 0 | Phosphorous Pentachloride \left(\text{PCl}_5\right) |
| Square Pyramidal | 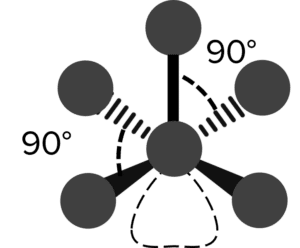 |
90\degree | 1 | Chlorine Pentafluoride \left(\text{ClF}_5\right) | |
| 6 | Octahedral | 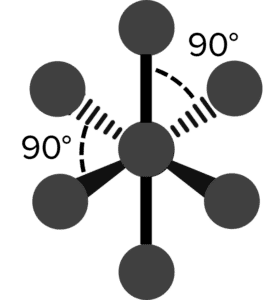 |
90\degree | 0 | Sulfur Hexafluoride \left(\text{SF}_6\right) |
Example: Bond angles
Draw the shapes of the ammonia molecule and the amide ion. You should include any lone pairs in your diagrams. You should indicate on the diagrams the bonding angles with in the molecules, as well as the charge on the amide ion.
[8 marks]
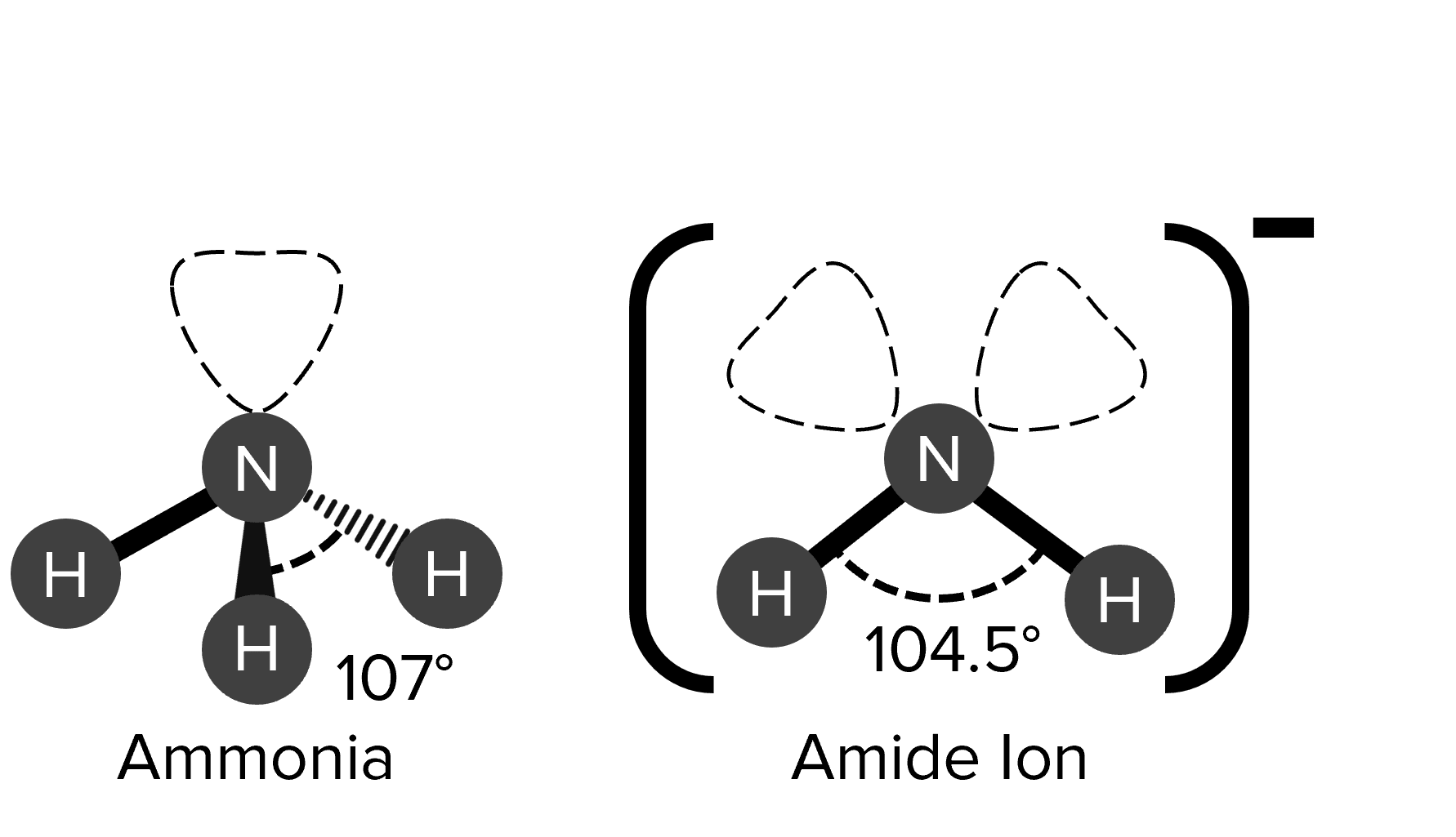
1 mark per correctly drawn structure (ammonia: trigonal pyramid, amid ion: bent).
1 mark for lone pair on ammonia.
1 mark per lone pair on the amide ion.
1 mark per correct bonding angle (ammonia: 107\degree, amide ion: 104.5\degree).
1 mark for negative (-) charge on amide ion.
Bonding and Structure Example Questions
Question 1: Why does Sodium (\text{Na}) have a higher melting point than Magnesium (\text{Mg})?
[4 marks]
- Magnesium has stronger metallic bonding.
- Magnesium has more delocalised electrons.
- Magnesium forms a smaller ion.
- So stronger electrostatic forces of attraction between ions and electrons so more energy is needed to break bonds.
Question 2: Draw the shapes, including lone pairs, of a phosphine (\text{PH}_3) molecule. Name the shape of phosphine and the bonding angle of the phosphine molecule.
[2 marks]
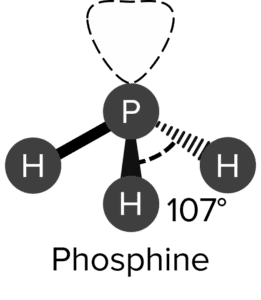
Trigonal pyramid
107\degree
Question 3: Explain why iodine, in solid form, vaporises when gently warmed.
[2 marks]
- Iodine has weak intermolecular forces.
- So it requires little energy to break the forces.
Question 4: State how a dative covalent bond is formed.
[2 marks]
- Both electrons/ electron pair
- Are donated from one atom/molecule/ species
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.