Group 2 Trends and Reactions
Group 2 Trends and Reactions Revision
Group 2 – Trends and Reactions
Group 2 elements are known as alkaline earth metals and all have two electrons in their outer shell. Group 2 metals undergo a range of reactions and have different uses in industry.
Group 2 Trends
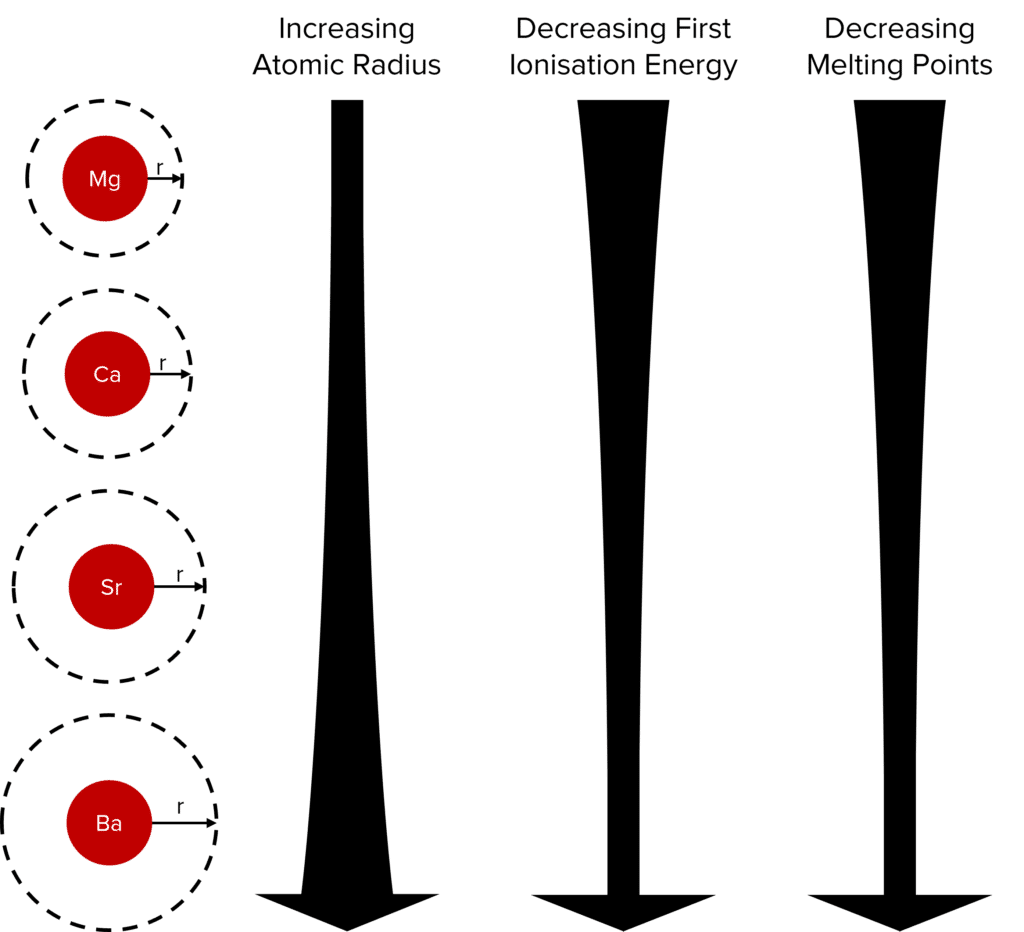

Berylium, magnesium, calcium and barium are a few examples of group 2 elements. All group 2 elements have two electrons in their outermost shell which allows them to exhibit similar chemical properties.
Atomic Radius
Atomic radius increases down group 2. Although all atoms in group 2 have two electrons in their outer shell, going down the group the number of electron shells increases by one. This increases the distance and shielding between the outer electrons and the nucleus, decreasing the electrostatic attraction between them.
First Ionisation Energy
First ionisation energy decreases down the group because the outermost electrons are further from the nucleus, so less energy is required for an electron to be removed. There is also increased shielding going down group 2 which decreases the attraction of the outer electron to the nucleus.
Melting Points
Melting point decreases down the group because the metallic bonds become weaker as the size of the atoms increase. The greater the distance between the delocalised electrons and positive ions, the weaker the electrostatic forces of attraction.
Reactions with Oxygen
The Group 2 metals will all react with oxygen to form metal oxides. These reactions are typically very slow however. Though the Group 2 metals will burn in oxygen they are typically quite difficult to ignite.
The formation of metal oxides tends to follow the general formula:
2\text{M}+\text{O}_2\rarr2\text{MO}
Magnesium is particularly easy to ignite and will burn with a bright white flame. This leads to the formation of magnesium oxide \left(\text{MgO}\right), a white powder.
2\text{Mg}+\text{O}_2\rarr2\text{MgO}
Without a flame, this reaction happens very slowly. The \text{MgO} in this case is formed as a thin layer that covers the surface of the magnesium. If the metal is to be used in processes like electrolysis, it must be rubbed down with emery paper to remove this oxide layer.
Reactions with Water
Group 2 metals will, for the most part, react very vigorously with water. These reactions will lead to the formation of hydrogen gas and of metal hydroxides.
\text{M}+\text{H}_2\text{O}\rarr\text{M}\left(\text{OH}\right)_2+\text{H}_2
Some metal hydroxides will be soluble and so stay in solution. In these cases, an alkaline aqueous solution will be formed from the reaction.
Magnesium is the least reactive of the Group 2 metals and so will only react with warm water:
\text{Mg}_{\text{(s)}} + 2\text{H}_2\text{O}_{\text{(l)}} \rarr \text{Mg(OH)}_{2\text{(aq)}} + \text{H}_{2\text{(g)}}
This reaction is fairly tame and does not produce a flame.
If magnesium reacts with steam, it forms magnesium oxide and hydrogen. This reaction does produce a flame, with magnesium burning with a bright white colour:
\text{Mg}_{\text{(s)}} + \text{H}_2\text{O}_{\text{(g)}} \rarr \text{MgO}_{\text{(s)}} + \text{H}_{2\text{(g)}}
Other elements in group 2 can form hydroxides by reacting with cold water since they are more reactive:
\begin{aligned}\text{Ca}_{\text{(s)}} + 2\text{H}_2\text{O}_{\text{(l)}}& \rarr \text{Ca(OH)}_{2\text{(s)}} + \text{H}_{2\text{(g)}}\\\text{Sr}_{\text{(s)}} + 2\text{H}_2\text{O}_{\text{(l)}}& \rarr \text{Sr(OH)}_{2\text{(aq)}} + \text{H}_{2\text{(g)}}\\\text{Ba}_{\text{(s)}} + 2\text{H}_2\text{O}_{\text{(l)}}& \rarr \text{Ba(OH)}_{2\text{(aq)}}+ \text{H}_{2\text{(g)}}\end{aligned}
Beryllium is the only group 2 metal that does not react with water.
The reactions of these group 2 metals are characterised by some common observations:
- An increase in the temperature of the reaction (exothermicity).
- The metal dissolving.
- The melting of the metal into a ball.
- The metal moving around on the surface of the water.
- The ignition of the metal, which burns with a coloured flame.
- Effervescence (fizzing created by the production of hydrogen gas) .
As you descend the group and the reactivity of the metals increases, the reactions become more vigorous. As such the above observations become more prominent. As we get towards the bottom of the group, the ignition of the metals will also cause the hydrogen produced in the reaction to ignite. This means that, as the metals become more reactive, the reactions become more explosive.
The Extraction of Titanium
Titanium is not a Group 2 metal. However, it can be extracted from its ores using the Group 2 metal magnesium. Titanium is used in aircrafts and in hip replacements for its resistance to corrosion, abundance, and a low density. Although relatively abundant, titanium is an expensive metal so is only used in small amounts.
The main factors that drive up the price of titanium are:
- High temperatures are needed in the extraction.
- The extraction process also requires an argon environment which is expensive.
- Magnesium is also required for extraction, which is expensive
- Extracting titanium is a batch process, which means that a lot of labour is required and energy is lost whenever the process stops.
The Extraction of Titanium
Titanium is extracted through a reaction with a more reactive metal, usually magnesium. Neither electrolysis nor reduction with carbon can be used to extract titanium from its ore titanium oxide \left(\text{TiO}_2\right). Reduction by carbon cannot be used as this leads to the production of titanium carbide instead of titanium metal. Electrolysis cannot be used as titanium as it not reactive enough.
The extraction of titanium using magnesium follows the following process:
1. Solid titanium oxide \left(\text{TiO}_2\right) is converted to liquid titanium chloride \left(\text{TiCl}_4\right) at a temperature of 9000\degree \text{C}.
\text{TiO}_{2\text{(s)}} + 2\text{Cl}_{2\text{(g)}} + 2\text{C}_{\text{(s)}}\rarr \text{TiCl}_{4\text{(l)}} + 2\text{CO}_{\text{(g)}}
This is done to allow for purification as \text{TiCl}_4 is liquid at room temperature.
2. The liquid titanium chloride is then purified by fractional distillation.
3. Magnesium is added to the liquid titanium chloride to extract solid titanium. As magnesium is more reactive than titanium, it will displace it in the salt to form magnesium chloride:
\text{TiCl}_{4\text{(l)}} + 2\text{Mg}_{\text{(s)}}\rarr \text{Ti}_{\text{(s)}} + 2\text{MgCl}_{2\text{(l)}}
Group 2 Trends and Reactions Example Questions
Question 1: Write an equation for the reaction of barium with water. (State symbols are not required.)
[1 mark]
\text{Ba}+2\text{H}_2\text{O}\rarr\text{Ba(OH)}_2+\text{H}_2
Question 2: Magnesium ribbon was used in a lab experiment with \text{HCl}. The ribbon was left unsealed and was exposed to air. Explain why it is important to clean the magnesium ribbon before using it in a reaction with \text{HCl}.
[2 marks]
- There is a thin layer of \text{MgO}.
- If not removed the \text{MgO} will react and not the magnesium OR Magnesium and Magnesium Oxide react at different rates.
Question 3: Give two conditions used in the production of titanium and state why carbon is not used in its extraction.
[3 marks]
Conditions:
- Heat
- Argon/ inert atmosphere
Reason:
- Titanium carbide is formed instead of elemental titanium
Question 4: Magnesium ribbon reacts with hot water while heated magnesium ribbon reacts with steam. Give two differences between these two reactions.
[2 marks]
Any two from:
- Bright white light when Mg reacts with steam.
- The reaction is slower with hot water OR the reaction is faster with steam.
- Hot water produces Magnesium hydroxide while steam produces magnesium oxide.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.