Group 2 Solubility and Chemical Tests
Group 2 Solubility and Chemical Tests Revision
Group 2 – Solubility & Tests
Group 2 elements are known as alkaline earth metals. Group 2 metals can form sulfates and hydroxides that have various uses.
Solubility of Group 2 Hydroxides
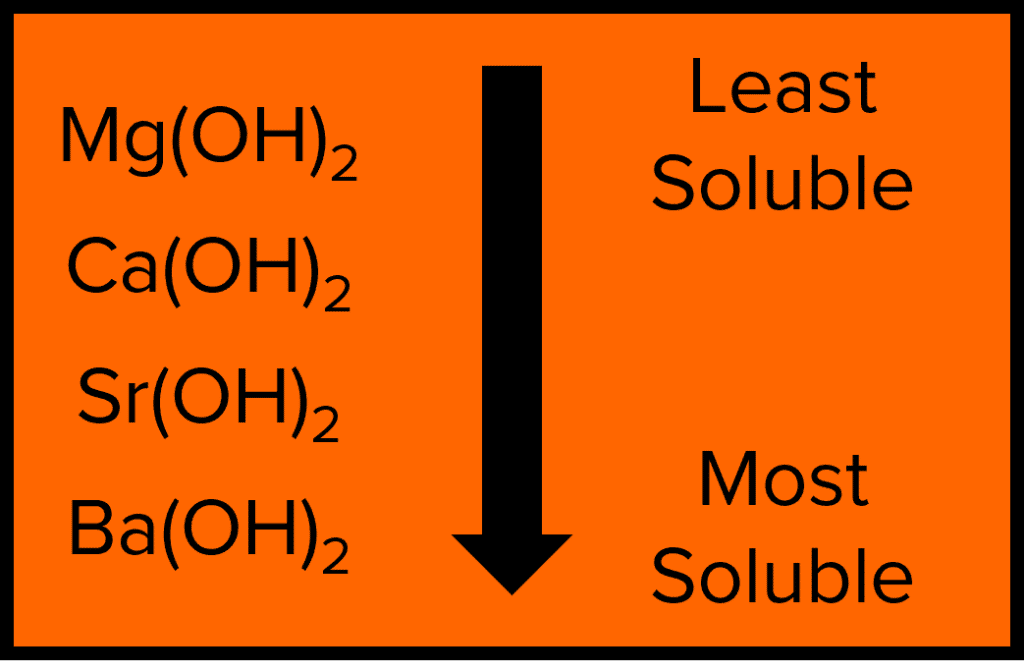

The Group 2 elements are able to form metal hydroxides when reacting with water. These hydroxides display a trend in their solubility in water. The solubility of the Group 2 Metal hydroxides increases as as we go down the group from magnesium to barium. The solubility of these hydroxides increases due to the increasing radius of the metal cations.
Ions that are similar in size will experience stronger electrostatic attractions to one another as they are able to get closer together. As the hydroxide ion \left(\text{OH}^-\right) is quite small, smaller metal cations will create stronger and so less soluble ionic compounds.
Group 2 metal hydroxides are all basic compounds. When dissolved in solution they will form alkaline solutions, with the alkalinity of the solutions increasing down the group as solubility increases. This means that they are able to react with and neutralise acids. These neutralisation reactions are used for a range of purposes, from agriculture to medicine.
Magnesium Hydroxide
Though magnesium hydroxide is considered insoluble in water, in a solution of magnesium hydroxide and water, the solution will become slightly alkaline. This solution will be about \text{pH }9, meaning that some hydroxide ions are present. Therefore, in reality, magnesium hydroxide is referred to as being ‘sparingly’ soluble.
\text{Mg(OH)}_{2\text{(s)}} \rarr \text{Mg}^{2+}_{\text{ (aq)}} + 2\text{OH}^{-}_{\text{ (aq)}}
Magnesium hydroxides lack of solubility makes it ideal for use in anti-acid medications. Usually taken in the form of a chewable tablet, \text{Mg(OH)}_2 reaches the stomach as a solid, where upon it neutralises with excess stomach acid.
Calcium Hydroxide
Calcium hydroxide is partially soluble in water, producing an alkaline solution \left(\text{pH }11\right).
Aqueous solutions of calcium hydroxide (limewater) can be used to test for carbon dioxide. In this test, \text{CO}_2 is bubbled through limewater causing calcium carbonate to be formed which presents as a white precipitate.
\text{Ca(OH)}_{2\text{(aq)}} + \text{CO}_{2\text{(g)}} \rarr \text{CaCO}_{3\text{(s)}} + \text{H}_2\text{O}_{\text{(l)}}
Calcium carbonate itself is used in the the removal of sulfur dioxide from flue gasses. A slurry of \text{CaCO}_3 is sprayed into the gasses and reacts reacts with acidic sulfur dioxide to produce solid calcium sulfite.
Calcium hydroxide is also used in agriculture to maintain soil quality. When soil becomes too acidic for a crop, \text{Ca(OH)}_2 can be added to increase its \text{pH}.
Barium Hydroxide
Barium hydroxide is very soluble in water. This means that a solution of barium hydroxide and water is very strongly alkaline.
\text{Ba(OH)}_{2\text{(s)}} \rarr \text{Ba}^{2+}_{\text{ (aq)}} + 2\text{OH}^{-}_{\text{ (aq)}}
Solubility of Group 2 Sulfates
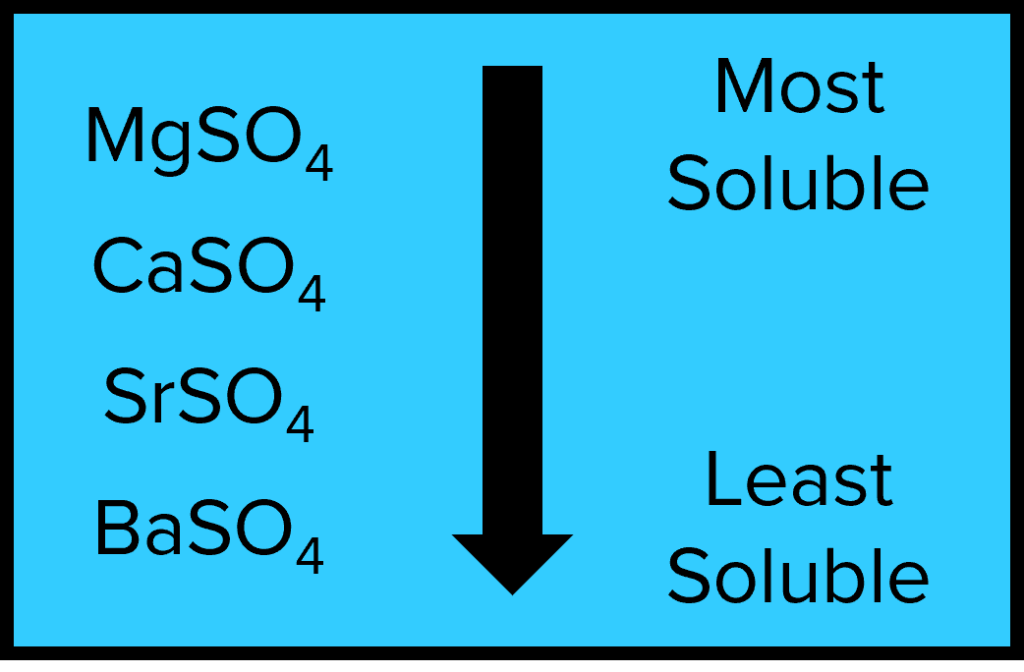

The Group 2 metals are also able to form metal sulfates upon reaction with sulfuric acid. The solubility of the metal sulfates follows the opposite trend to the metal hydroxides. The metal sulfates become less soluble down the group for the same reason as the increasing solubility of the of the metal hydroxides. In this case, the sulfate ion is large and so larger cations form stronger ionic compounds.
The group 2 metal sulfates are less commonly used, though barium sulfate is used in medicine.
Barium Sulfate
The insoluble nature of \text{BaSO}_4 means that it is ideal for use as a contrast medium in certain types of x-ray. The solid barium sulfate will move through out the digestive system without being absorbed and shows up dark on an x-ray, allowing doctors to locate blockages.
The insolubility of \text{BaSO}_4 means that barium can also be used to test for sulfate ions in solution. When barium chloride is added to a solution containing sulphate ions, the chloride ions will be displaced by the sulfate ions. This produces solid insoluble barium sulfate, which falls to the bottom of the reaction as a white precipitate:
\text{BaCl}_{2\text{(aq)}}+\text{SO}_{4\text{(aq)}}^{ 2-}\rarr\text{BaSO}_{4\text{(s)}}+2\text{Cl}^{-}_{\text{(aq)}}
Barium reacts slowly with sulfuric acid because solid barium sulfate forms on the surface of the barium metal, slowing down the reaction.
Group 2 Solubility and Chemical Tests Example Questions
Question 1: State a medical use of barium sulfate. Suggest barium sulfate is safe for this use.
[2 marks]
- Used in a barium meal.
- Barium sulfate is insoluble.
Question 2: Magnesium hydroxide and magnesium carbonate can be used to reduce acidity in the stomach.
Other than cost, explain one advantage of using magnesium hydroxide rather than magnesium carbonate to reduce acidity in the stomach.
[1 mark]
Magnesium hydroxide does not produce \text{CO}_2.
(Allow reference to low reactivity of magnesium)