Halogens Trends and Properties
Halogens Trends and Properties Revision
Halogens Trends and Properties
Elements found in group 7 of the periodic table are known as halogens. Halogens show very interesting trends in properties such as melting points and electronegativity. They can also undergo displacement reactions.
Introduction to Halogens
Halogens are non-metallic elements found in group 7 of the periodic table. The first four halogens in the periodic table are fluorine, chlorine, bromine, and iodine. Each of these halogens has a different colour that allows it to be easily identified. These colours generally become darker as you go down the group.
| Halogen | Physical State at Room Temperature \left(20\degree\text{C}\right) | Colour |
| Fluorine \left(\text{F}_2\right) | Gas | Pale Yellow |
| Chlorine \left(\text{Cl}_2\right) | Gas | Pale Green |
| Bromine \left(\text{Br}_2\right) | Liquid | Dark Orange Liquid, Orange Vapour |
| Iodine \left(\text{I}_2\right) | Solid | Grey Solid, Purple Vapour |
Trends in Halogens
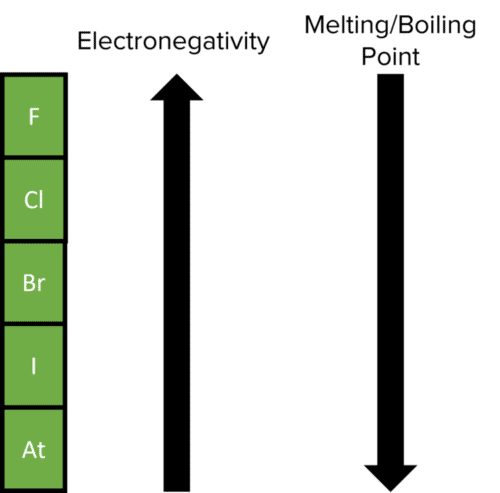
Halogens all have seven electrons in their outer shell so they show very similar chemical properties and have trends as you move up or down the group. Two particular trends that you need to know about are electronegativity and melting/boiling point.
Electronegativity
Electronegativity decreases down the group. This is because the atomic radii of the elements increase down the group, so the attraction between the nucleus and outer electrons decreases.
Melting/ Boiling Point
The melting and boiling point of the halogens increases down the group. This is because the molecules are larger further down the group, so the electron density increases and therefore, there are increased Van der Waals forces.
These increased Van der Waals forces mean that more energy is needed to break the attraction between molecules, and so melting/ boiling point increases.
Displacement Reactions
Halogens can react with metals to form ions with a -1 charge such as \text{I}^-. They form these negative ions by oxidising metals to form positive metal ions (making them oxidising agents). These oppositely charged ions will then come together to form ionic substances. These ionic substances can then undergo displacement reactions with other halogens. A displacement reaction is one in which a more strongly oxidising halogen replaces a weaker one in an ionic compound.
The oxidising strength of different halogens will determine which halogen replaces which in a displacement reaction For example, chlorine is able to displace bromine in ionic compunds:
2\text{NaBr}+\text{Cl}_2\rarr2\text{NaCl}+\text{Br}_2
- Chlorine has a higher oxidising strength then bromine.
- Chorine can therefore displace bromine \text{NaBr}
- As such, upon reaction, an orange solution of bromine is formed.
| Metal Halide | Halogen Added | ||
| Chlorine \left(\text{Cl}_2\right) | Bromine \left(\text{Br}_2\right) | Iodine \left(\text{I}_2\right) | |
| Sodium Chloride \left(\text{NaCl}\right) | No reaction, solution remains orange. | No reaction, solution remains brown. | |
| Sodium Bromide \left(\text{NaBr}\right) |
Orange solution formed \text{Cl}_{2\text{(aq)}}+2\text{Br}^{-}_{\text{(aq)}}\rarr2\text{Cl}^{-}_{\text{(aq)}}+\text{Br}_{2\text{(aq)}} |
No Reaction, solution remains brown. |
|
| Sodium Iodide \left(\text{NaI}\right) |
Brown solution formed \text{Cl}_{2\text{(aq)}}+2\text{I}^{-}_{\text{(aq)}}\rarr2\text{Cl}^{-}_{\text{(aq)}}+\text{I}_{2\text{(aq)}} |
Brown solution formed \text{Br}_{2\text{(aq)}}+2\text{I}^{-}_{\text{(aq)}}\rarr2\text{Br}^{-}_{\text{(aq)}}+\text{I}_{2\text{(aq)}} |
|
Identifying Halide Ions
Halide ions form precipitates of different colours when they undergo certain reactions, making them identifiable in solution.
To identify halide ions, an acidified silver nitrate solution \left(\text{AgNO}_3\right) is added to the solution containing halide ions. Nitric acid is first added to react with any carbonates, hydroxides, or sulfites in the silver nitrate solution so that they will not form a precipitate that would interfere with the colour of the precipitates.
The reactions that take place and the precipitates formed are shown in the table below:
| Halide Ion | Addition of Silver Nitrate | |
| Reaction | Precipitate | |
| Fluoride \left(\text{F}^{-}\right) | \text{Ag}^{+}_{\text{(aq)}}+\text{F}^{-}_{\text{(aq)}}\rarr\text{AgF}_{\text{(aq)}} | No precipitate. Silver fluoride is soluble in water. |
| Chloride \left(\text{Cl}^{-}\right) | \text{Ag}^{+}_{\text{(aq)}}+\text{Cl}^{-}_{\text{(aq)}}\rarr\text{AgCl}_{\text{(s)}} | A white precipitate is formed. |
| Bromide \left(\text{Br}^{-}\right) | \text{Ag}^{+}_{\text{(aq)}}+\text{Br}^{-}_{\text{(aq)}}\rarr\text{AgBr}_{\text{(s)}} | A cream precipitate is formed. |
| Iodide \left(\text{I}^{-}\right) | \text{Ag}^{+}_{\text{(aq)}}+\text{I}^{-}_{\text{(aq)}}\rarr\text{AgI}_{\text{(s)}} | A pale yellow precipitate is formed. |
Although the precipitates formed by the different halide ions are different colours it can sometimes be challenging to differentiate between the different colours formed by the silver halides, so ammonia is also added to differentiate them.
Dilute ammonia can be used to clearly identify \text{Cl}^{-} ions since the precipitate dissolves. Concentrated ammonia can be used to clearly identify \text{I}^{-} ions as the precipitate is the only one that does not dissolve.
| Silver Halide | Addition of Dilute Ammonia | Addition of Concentrated Ammonia | ||
| Reaction | Precipitate | Reaction | Precipitate | |
| \text{AgF} | No reaction. | No precipitate | No reaction | No precipitate. |
| \text{AgCl} |
Silver chloride is dissolved by dilute ammonia. |
Silver chloride precipitate dissolves into solution. | No reaction | Silver chloride precipitate dissolves into solution. |
| \text{AgBr} | No Reaction. Silver bromide is insoluble in dilute ammonia. | Cream precipitate formed. |
Silver bromide is dissolved by concentrated ammonia. |
Silver bromide precipitate dissolves into solution. |
| \text{AgI} | No Reaction. Silver iodide is insoluble in dilute ammonia. | Yellow precipitate formed. | No reaction. Silver iodide is insoluble in concentrated ammonia. | Yellow precipitate formed. |
Halogens Trends and Properties Example Questions
Question 1: Define an oxidizing agent.
[1 mark]
A species that accepts electrons.
Question 2: State and explain the trend in electronegativity for the elements down group 7.
[3 marks]
- Electronegativity decreases down the group.
- Because atomic radii increases (shielding increases/ number of shells increase).
- So there is a weaker attraction between the nucleus and outer electrons.
Question 3: Chlorine displaces iodine from aqueous potassium iodide.
a.) Write the simplest ionic equation for this reaction.
b.) Give one observation that would be made when this reaction occurs.
[2 marks]
a.)
\text{Cl}_2+2\text{I}^{-}\rarr 2\text{Cl}^{-}+\text{I}_2
b.)
The solution becomes brown.
Question 4: A yellow precipitate is formed when a silver nitrate solution, acidified with dilute nitric acid, is added to an aqueous solution containing iodide ions.
a.) State what is observed when concentrated ammonia is added to the yellow precipitate.
b.) State why the silver nitrate solution is acidified when testing for iodide ions.
[2 marks]
a.)
The precipitate does not dissolve/ is insoluble.
b.)
To remove ions that would interfere with the test ( remove carbonates/ hydroxides/ sulfites OR prevent the formation of other silver precipitates).
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.