Empirical & Molecular Formulae
Empirical & Molecular Formulae Revision
Empirical & Molecular Formulae
The empirical formula of a compound is the simplest ratio of atoms in a compound while the molecular formula is the actual number of atoms of each element. By calculating the empirical formula of a compound, we can calculate its molecular formula using the empirical and molecular masses.
Calculating the Empirical Formula
To calculate the empirical formula:
- Find the moles of each element. This can be done by dividing the mass (or percentage mass) by the atomic mass.
- Divide each of the moles by the smallest number of moles calculated.
- Make sure that each of the numbers are integers.
Example: Calculate the empirical formula for a compound that contains 5.14\text{ grams} of Carbon, 0.86\text{ grams} of Hydrogen and 6.89\text{ grams} of Oxygen.
\text{Moles of C} = \dfrac{5.14}{12}=0.428\text{ Moles of H} = \dfrac{0.86}{1}=0.86\text{ Moles of O} = \dfrac{6.89}{16}=0.431
0.428 is the smallest moles calculated so all the moles will be divided by it.
\text{C:} \dfrac{0.428}{0.428} =1\text{ H:} \dfrac{0.86}{0.428} \approx2\text{ O:} \dfrac{0.431}{0.428} \approx1
Empirical Formula – \text{CH}_2\text{O}
Calculating the Molecular Formula
To calculate the molecular formula, we use the mass of the empirical formula and \text{M}_{\text{r}} to determine how many times the mass can be divided into the \text{M}_{\text{r}}.
Example: Deduce the molecular formula for the compound with the empirical formula \text{C}_2\text{H}_4\text{O} and has an \text{M}_{\text{r}} of 88.
\text{Mass of C}_2\text{H}_4\text{O is }44
\dfrac{88}{44}= 2
So the empirical formula should be multiplied by 2.
\text{C}_4\text{H}_8\text{O}_2
Required Practical
Hydrated Salts
This practical is used to measure the loss or gain of mass in different reactions such as thermal decomposition.
Water of Crystallisation is the water contained within the crystals of a hydrated salt.
In this experiment, calcium sulfate crystals are heated to remove water vapour so that the water of crystallisation in the compound can be calculated.
The equation for the reaction that takes place is \text{CaSO}_4\text{.xH}_2\text{O}_{\left(\text{s}\right)}\rightarrow\text{CaSO}_{4\left(\text{s}\right)}+\text{xH}_2\text{O}_{\left(\text{g}\right)}.
- Weigh an empty and dry crucible along with the lid.
- Add 2\text{ g} of hydrated calcium sulfate to the crucible and weigh it again.
- Heat the crucible strongly for a few minutes and then leave it to cool.
- Weigh the crucible and its contents again.
- Repeat steps 3-4 until the mass is constant. This would mean that the reaction is complete.
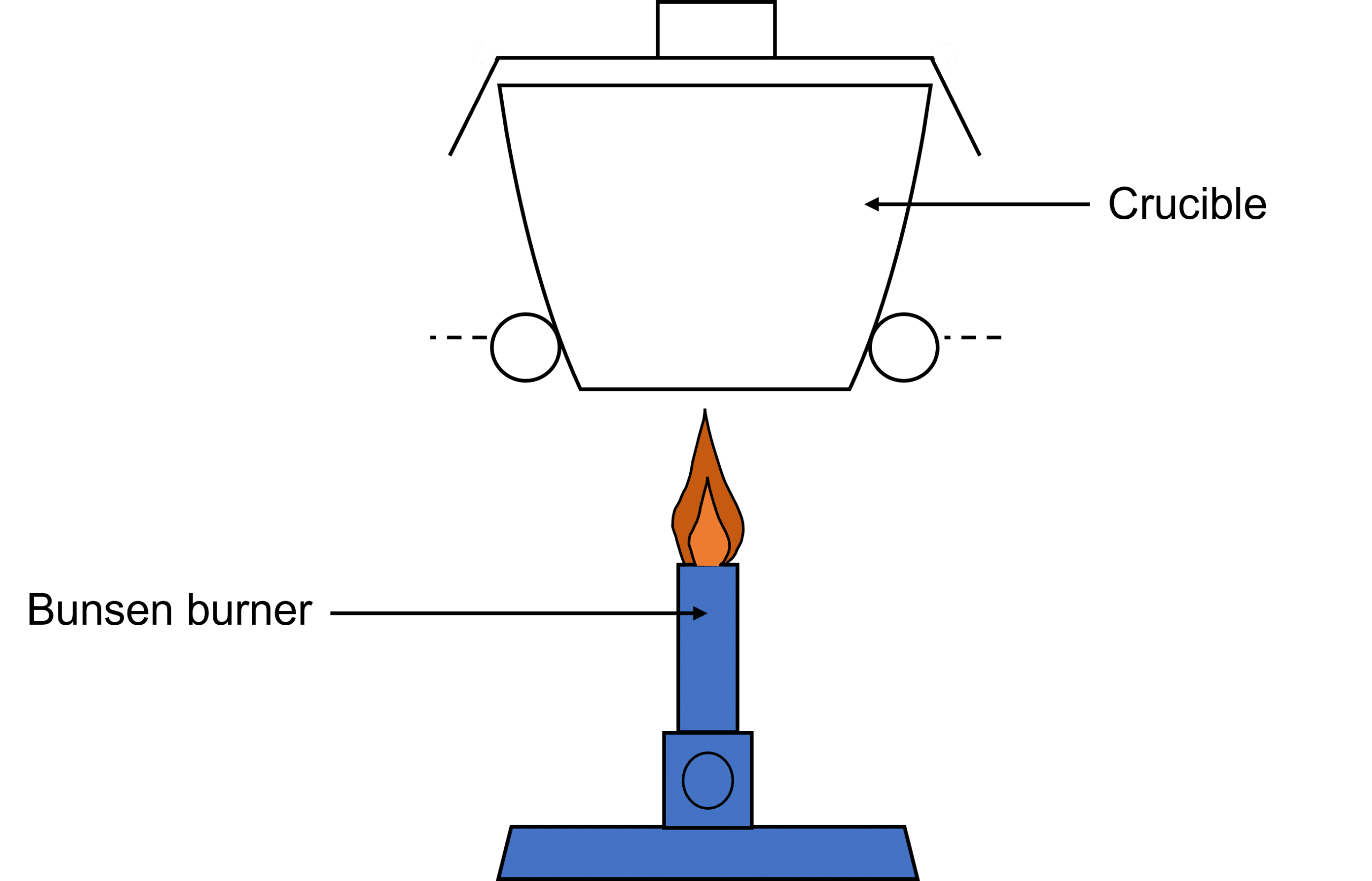
Note: When carrying out experiments in a crucible, you should not use small or large amounts of solid. If using small amounts, the weights taken will not be as accurate because of the high percentage uncertainty. For bigger amounts, there will likely be incomplete decomposition.
Water of Crystallisation Calculations
The water of crystallisation is the water that becomes trapped in crystalline solids when they form in solution. This water will increase the mass of the compound in which it is trapped.
We indicate the water of crystallisation of a compound in it’s molecular formula using by writing the formula of water at the end of the compound’s formula:
\text{Na}_2\text{SO}_4\text{.H}_2\text{O}
A dot is written between the regular chemical formula and the water of crystallisation.
By heating a hydrated compound, you can work out the water of crystallisation within the compound.
Example: \textcolor{#00bfa8}{6.28\text{ g}} of sodium sulfate hydrate is heated. The mass after heating is [\textcolor{#f21cc2}{4.61\text{ g}}. Calculate the value of x in \text{Na}_2\text{SO}_4\text{.xH}_2\text{O}.
\text{Mass of H}_2\text{O}=\textcolor{#00bfa8}{6.28}-\textcolor{#f21cc2}{4.61}=\textcolor{#008d65}{1.67\text{ g}}
\text{Moles of Na}_2\text{SO}_4= \dfrac{\textcolor{#f21cc2}{4.61}}{142.1}=\textcolor{#008d65}{0.0324\text{ mol}}
\text{Moles of H}_2\text{O}=\dfrac{1.67}{18}=\textcolor{#008d65}{0.0928\text{ mol}}
\text{Ratio of Na}_2\text{SO}_4\text{H}_2\text{O}=\frac{0.0928}{0.324}\approx1:3
\text{X}=\textcolor{#008d65}{3}
Example: Calculating Empirical Formula
The percentage by mass for the elements of a compound are as follows: \textcolor{#00bfa8}{26.6\%} Potassium, \textcolor{#f21cc2}{35.4\%} Chromium and the remaining is Oxygen. Calculate the empirical formula for a compound.
[4 marks]
\text{Moles of K}= \dfrac{\textcolor{#00bfa8}{26.6}}{39.1}=0.680\text{ Moles of Cr}= \dfrac{\textcolor{#f21cc2}{35.4}}{52}=0.681\text{ Moles of O}= \dfrac{38}{16}=2.38
\text{K:} \dfrac{0.680}{0.680}=1\text{ Cr:} \dfrac{0.681}{0.680}\approx1\text{ O:} \dfrac{2.38}{0.680}\approx3.5
The ratio has to be whole numbers so the values need to be multiplied by 2.
\text{Kr}_2\text{Cr}_2\text{O}_7
Empirical & Molecular Formulae Example Questions
Question 1: A substance contains 43.7\text{ g} of phosphorus and 56.3\text{ g} of oxygen, its \text{M}_\text{r} is 284. Calculate the empirical and molecular formula of the substance.
[5 marks]
Step 1: Calculate the moles of phosphorus and oxygen.
\text{Moles of phosphorus}=\dfrac{43.7}{31}=1.4097
\text{Moles of oxygen}=\dfrac{56.3}{16}=3.5188
Step 2: Deduce the empirical formula of the substance.
\begin{aligned}&\text{P: }\frac{1.4097}{1.4097}&\text{ O:}\frac{3.5188}{1.4097}\\&= 1.0 &=2.5\end{aligned}
Simplest whole number ratio:
\left(\text{P: }1.0\times\text{ O:}2.5\right)\times2
\text{P}_2\text{O}_5
Step 3: Calculate the Molecular formula.
\text{M}_r =284
\text{Empirical Mass}=\left(2\times31\right)+\left(5\times16\right)=142
\text{Empirical Formula}:\text{Molecular Formula}=\frac{284}{142}=2
Molecular Formula: \text{P}_4\text{O}_{10}
(One mark for step 1. Two marks for step 2. Two marks for step 3.)
Question 2: 28.3\text{ g} of magnesium sulfate hydrate is heated. 13.7\text{ g} of anhydrous magnesium sulfate was obtained. Calculate the value of \text{x} in \text{MgSO}_4\text{.xH}_2\text{O}.
[4 marks]
\text{Mass of H}_2\text{O}= 28.3-13.7=14.6
\text{Moles of MgSO}_4 = \dfrac{13.7}{120.4}=0.11379
\text{Moles of H}_2\text{O}=\dfrac{14.6}{18}=0.81111
\text{Ratio of MgSO}_4 \text{ to H}_2\text{O}=\frac{0.81111}{0.11379}=7.13
\text{x}=7 \text{ OR MgSO}_4\text{.}7\text{H}_2\text{O}
Question 3: 14\text{ g} of hydrated nickel (II) nitrate is dried. 8.7\text{ g} of anhydrous nickel (II) nitrate remains. Find the value of \text{x} in \text{Ni}\left(\text{NO}_3\right)_2\text{.xH}_2\text{O}.
[4 marks]
\text{Mass of H}_2\text{O}=14-8.7=5.3 \text{g}
\text{Moles of Ni}\left(\text{NO}_3\right)_2=\dfrac{8.7}{182.7}=0.0476
\text{Moles of H}_2\text{O} = \dfrac{5.3}{18}= 0.2944
\text{Ratio of Ni}\left(\text{NO}_3\right)_2\text{ to H}_2\text{O}=\frac{2.944}{0.0476}=6.18
\text{x}=6 \text{ OR Ni}\left(\text{NO}_3\right)_2\text{.}6\text{H}_2\text{O}









