The Mole and Avogadro's Constant
The Mole and Avogadro's Constant Revision
The Mole and Avogadro’s Constant
One mole of a substance contains 6.02\times10^{23} particles. This number is known as Avogadro’s constant. Avogadro’s constant can be used to work out how many particles there are in a particular amount of moles.
Key Definitions
Relative Atomic Mass: The average mass of an atom of an element when compared to 1/12th of the mass of an atom of carbon-12.
Relative Molecular Mass: The average mass of a molecule when compared to 1/12th of the mass of an atom of carbon-12.
Moles, Mass and Mr
The number of moles of a substance can be calculated using the mass and molar mass (\text{M}_\text{r}) of the substance.
Equation:
\text{Moles}= \dfrac{\text{Mass}}{\text{M}_\text{r}}
\text{Moles: mol} \text{Mass: g} \text{M}_\text{r}\text{: g mol}^{-1}
Mr is calculated by adding up all the mass numbers of the elements in a molecule/ compound.
Example: Calculate the number of moles in 15.0\text{ g} of \text{KNO}_3.
\text{Moles}= \dfrac{\text{Mass}}{\text{M}_\text{r}}
\text{Moles}= \dfrac{15}{39.1+14+\left(16\times3\right)} = \dfrac{15}{101.1} = 0.148 \text{ mol}
Note: Always put your answer to the same number of significant figures as are in the question.
Unit Conversions
Sometimes, you may be given a mass that is not in grams and you will need to convert between the different units.
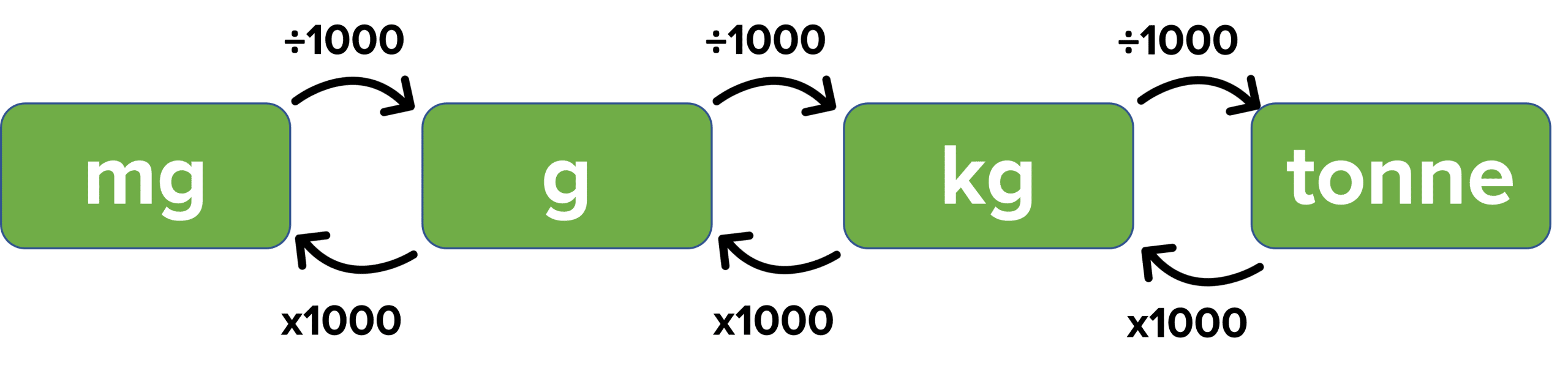
Example: Calculate the moles of \textcolor{#00bfa8}{83\text{ mg}} of \text{Mg}\left(\text{OH}\right)_{2}.
\begin{aligned}&\text{Mass in g} = \dfrac{\textcolor{#00bfa8}{83}}{1000}= \textcolor{#008d65}{0.083\text{ g}}\\&\text{Moles} =\dfrac{\text{Mass}}{\text{M}_{\text{r}}}\\ &\text{Moles} = \dfrac{0.083}{24.3+\left(2\times\left(16+1\right)\right)} = \dfrac{\textcolor{#00bfa8}{0.083}}{58.3}= \textcolor{#008d65}{1.4\times10^{-3}}\text{ mol}\end{aligned}
Avogadro’s Constant
Avogadro’s constant is the number of particles in one mole of substance.
\text{Avogadro's Constant}=6.02 \times10^{23}
The relationship between the number of moles, number of particles and Avogadro’s constant is represented in the equation:
\text{Moles} = \dfrac{\text{Number of particles}}{\text{Avogadro's Constant}}
Particles can be anything such as atoms, ions and molecules.
Example: Calculate the number of molecules in \textcolor{#00bfa8}{0.0056} moles of water.
\begin{aligned}\text{Moles} &= \dfrac{\text{Number of particles}}{\text{Avogadro's Constant}}\\\text{Number of particles}& = \text{Moles}\times\text{Avogadro's Constant}\\ \text{Number of particles} &= \textcolor{#00bfa8}{0.0056}\times6.02\times10^{23}\\ &= \textcolor{#008d65}{3.37\times10^{21}\text{ molecules}}\end{aligned}
Example 1: Calculating Mass of a Substance
Calculate the mass of \textcolor{#00bfa8}{0.036 \text{ moles}} of \text{NaCl}.
[2 marks]
\begin{aligned}&\text{Moles}= \dfrac{\text{Mass}}{\text{M}_\text{r}}\\&\text{Mass}=\text{Moles}\times\text{M}_\text{r}\\&\text{Mass}=\textcolor{#00bfa8}{0.036}\times\left(23+35.5\right) = 0.036\times58.5= \textcolor{#008d65}{2.11\text{ g}}\end{aligned}
Example 2: Calculating Moles of a Substance
Calculate the number of moles in \textcolor{#00bfa8}{28.6\text{ g}} of \text{CuSO}_{4}.5\text{H}_{2}\text{O}.
[2 marks]
\begin{aligned}\text{Moles}&= \dfrac{\text{Mass}}{\text{M}_\text{r}}\\&= \dfrac{\textcolor{#00bfa8}{28.6}}{63.5+32.1+\left(16\times4\right)+\left(5\times\left(2+16\right)\right)}\\&=\dfrac{\textcolor{#00bfa8}{28.6}}{249.6}\\&=\textcolor{#008d65}{0.115\text{ mol}}\end{aligned}
The Mole and Avogadro's Constant Example Questions
Question 1: Calculate the moles in 0.0347\text{ kg} of \text{Fe}_2\text{O}_3.
[3 marks]
\text{Mass in g}=0.0347\times1000=34.7\text{g}
\text{Moles} =\dfrac{\text{Mass}}{\text{M}_{\text{r}}}
\text{Moles}= \dfrac{34.7}{159.6}
\text{Moles}= 0.217\text{ mol}
Question 2: How many moles is 3.23 \times10^{23} atoms of \text{KCl}?
[2 marks]
\text{Moles} = \dfrac{\text{Number of particles}}{\text{Avogadro's Constant}}
\text{Moles} = \dfrac{3.23\times10^{23}}{6.02\times10^{23}}
\text{Moles} =0.536
Question 3: Find the number of atoms in 93\text{ mg} of \text{BaSO}_4.
[5 marks]
\text{Mass in g}= \dfrac{93}{1000}= 0.093\text{ g}
\text{Moles}=\dfrac{\text{Mass}}{\text{M}_{\text{r}}}= \dfrac{0.093}{233.4}
\text{Moles}= 3.98\times10^{-4}
\text{Moles} = \dfrac{\text{Number of particles}}{\text{Avogadro's Constant}}
\text{Number of atoms}= \text{Moles}\times\text{ Avogadro's Constant}
\text{Number of atoms}= 3.98\times10^{-4}\times6.02\times10^{23}= 2.4\times10^{20}
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.