The Ideal Gas Equation
The Ideal Gas Equation Revision
The Ideal Gas Equation
The ideal gas equation is an equation that can be used for gases and mixtures of gases. It relates the the amounts (moles and volume) of a gas to it’s physical conditions (pressure and temperature) through a physical constant (the ideal gas constant, R).
The Ideal Gas Equation
The ideal gas equation is an important formula that represents the relationship between a gases pressure, volume ,number of moles, temperature and the molar gas constant \left(8.31\text{J K}^{-1}\text{ mol}^{-1} \right).
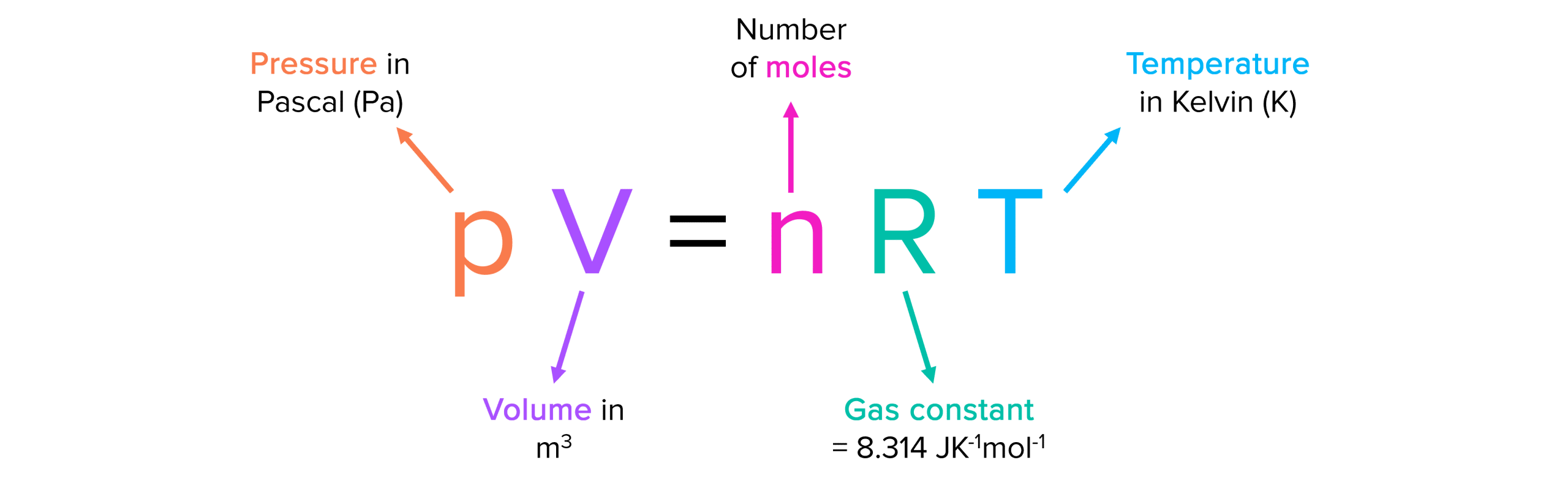
Sometimes, temperature needs to be converted from degrees Celsius \left(\text{°C}\right) to kelvin \left(\text{K}\right).
To do this you must add 273.
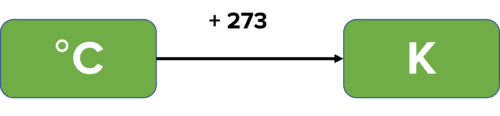
Gas Syringe
Gas syringes are usually used to measure the volume of gas produced in an experiment. The gasses produced are fed into a gas syringe by using a delivery tube. As the gas fills the syringe, it pushes back the stopper, allowing us to read off the volume of gas produced by the reaction. To prevent any loss of gas and to measure the most accurate possible volume, a bung should be placed in the top of the reaction vessel. The delivery tube is then inserted into the vessel through the bung.
Issues with Using Gas Syringes
- Some gas may escape before the bung is placed on the flask.
- The syringe may get stuck.
- Some gases are soluble in water so it is not possible to measure the true amount of gas.
Changing Conditions
In some questions, the same amount of a gas can be put under different conditions.
An equation can be used to carry out calculations involving changing conditions:
\dfrac{\text{p}_1\text{V}_1}{\text{T}_1}=\dfrac{\text{p}_2\text{V}_2}{\text{T}_2}
Example: At 300\text{K} and \textcolor{#00bfa8}{100\text{ kPa}}, \textcolor{#f21cc2}{80 \text{ cm}^3} of oxygen and \textcolor{#a233ff}{120\text{ cm}^3} of carbon dioxide are placed into a \textcolor{#eb6517}{1.5\text{ dm}^3} evacuated flask. Calculate the pressure of the gas mixture at 300\text{K} in \text{kPa}.
First we calculate the value of \text{V}_1.
\text{V}_1=\textcolor{#a233ff}{0.120\text{ dm}^3} + \textcolor{#f21cc2}{0.080\text{ dm}^3}=\textcolor{#008d65}{0.200\text{ dm}^3}
Now we calculate the value of \text{p}_2
\dfrac{\text{p}_1\text{V}_1}{\text{T}_1}=\dfrac{\text{p}_2\text{V}_2}{\text{T}_2}
We can cancel out the temperature since it does not change.
\text{p}_1\text{V}_1=\text{p}_2\text{V}_2
Then rearrange the equation to make \text{p}_2 the subject and substitute in the numbers from the question.
\begin{aligned}\text{p}_{2}&= \dfrac{\text{p}_1\text{V}_1}{\text{V}_2}\\&=\dfrac{\textcolor{#00bfa8}{100000}\times2\times10^{-4}}{1.5\times10^{-3}}\\&=13333\text{ Pa}\\&=\textcolor{#008d65}{13\text{ kPa}}\end{aligned}
Note: The variables in the last equation have been converted to SI units before subsitution.
Reacting Volumes of Gas
1 \text{ mol} of any gas at room temperature \left(25\text{°C}\right) and pressure \left(1 \text{ atm}\right) will have a volume of 24\text{ dm}^3.
In the same temperature and pressure, equal volumes of gases have an equal number of moles.
Due to this, the volume of gases reacting can be calculated by using ratios in the balanced equation.
Example: 730\text{ cm}^3 of propane is combusted at 1\text{ atm} and 300\text{ K}. Calculate the volume of oxygen needed to react under the same conditions.
\text{C}_3\text{H}_8 + 5\text{O}_2 \rightarrow 3\text{CO}_2 + 4\text{H}_2\text{O}
\text{Ratio of Propane to Oxygen}- 1:5
\text{Volume of oxygen}= 730\times5= 3650\text{cm}^3
Example 1: pV=nRT
Calculate the moles of \text{N}_2 gas at a pressure of \textcolor{#00bfa8}{100\text{kPa}}, temperature \textcolor{#f21cc2}{30\text{ °C}}, volume \textcolor{#a233ff}{450\text{ cm}^3}.
The molar gas constant is 8.31\text{ J K}^{-1}\text{ mol}^{-1}
[3 marks]
\begin{aligned}\text{pV}&=\text{nRT}\\\text{n}&=\dfrac{\text{pV}}{\text{RT}}\\&=\dfrac{\textcolor{#00bfa8}{100000}\times\textcolor{#a233ff}{0.00045}}{8.31\times\textcolor{#f21cc2}{303}}\\&=\textcolor{#008d65}{0.0178\text{ mol}}\end{aligned}
Example 2: pV=nRT
The moles of gas in a flask of \textcolor{#00bfa8}{12\text{dm}^3} is \textcolor{#f21cc2}{0.725}. Use the ideal gas equation to determine the temperature \left(\text{T}\right) in \text{°C}, at which the pressure in the flask is \textcolor{#a233ff}{3.3\times10^5\text{Pa}}.
[4 marks]
\begin{aligned}\text{pV}&=\text{nRT}\\\text{T}&=\dfrac{\text{pV}}{\text{nR}}\\&=\dfrac{\textcolor{#a233ff}{3.3\times10^5}\times\textcolor{#00bfa8}{12\times10^{-3}}}{\textcolor{#f21cc2}{0.724}\times8.31}\\&=657\text{K}\\&=\textcolor{#008d65}{384\text{°C}}\end{aligned}
The Ideal Gas Equation Example Questions
Question 1: A 1.48\text{g} sample of ethanol vapour \left(\text{C}_2\text{H}_5\text{OH}\right) is at a pressure of 100\text{ kPa} and a temperature of 95\text{ °C}. Calculate the volume, in \text{cm}^3, occupied by the ethanol vapour under these conditions.
[5 marks]
\text{Number of moles}=\dfrac{1.48}{46}= 0.03217
\text{p}= 100000\text{ Pa and T}= 368\text{ K}
\begin{aligned}\text{V}&=\dfrac{\text{nRT}}{\text{p}}\\ &= \dfrac{0.03217\times8.31\times368}{100000}\\&=9.84\times10^{-4}\text{ m}^{3}\\=984\text{ cm}^3
Question 2: In the reaction, 4\text{NH}_{3\left(\text{g}\right)}+5\text{O}_{2\left(\text{g}\right)}\rightarrow4\text{NO}_{\left(\text{g}\right)}+6\text{H}_2\text{O}_{\left(\text{g}\right)}, the gases formed occupy a total volume of 5.07\text{ m}^3 at 25\text{ °C} and 100\text{ kPa}.
Calculate the amount, in moles, of \text{NO} produced.
(The molar gas constant, R = 8.31\text{ J K}^{-1}\text{ mol}^{-1})
[4 marks]
\text{P}=100000\text{Pa and T}=298\text{ K}
\begin{aligned}\text{n}&=\dfrac{\text{pV}}{\text{RT}}\\&=\dfrac{100000\times5.07}{8.31\times298}\\&=205\text{ mol}\end{aligned}
\text{Moles of NO}=\dfrac{4}{10}\times 205=81.9\text{ mol}
Question 3: Sodium azide decomposes to form sodium and nitrogen.
2\text{NaN}_{3\left(\text{s}\right)}\rightarrow2\text{Na}_{\left(\text{s}\right)}+3\text{N}_{2\left(\text{g}\right)}
The nitrogen fills a volume of 7.80\times10^{-2}\text{ m}^{3} at a pressure of 150\text{ kPa} and a temperature of 30\degree \text{C}.
Calculate the minimum mass of sodium azide decomposed in this reaction.
[6 marks]
\text{P}= 150000\text{ Pa and T}= 303\text{ K}
\begin{aligned}\text{n}&=\dfrac{\text{pV}}{\text{RT}}\\&=\dfrac{150000\times7.8\times10^{-2}}{8.31\times303}\\&=4.6467\text{ mol}\end{aligned}
\text{Moles of NaN}_3=\dfrac{2}{3}\times4.6467=3.098\text{ mol}
\begin{aligned}\text{Mass of NaN}_3&=3.098\times65\\&=201 \text{ g}\end{aligned}
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.