The Haber Process
The Haber Process Revision
The Haber Process
The Haber process is used to produce ammonia. It takes advantage of the equilibrium that exists between hydrogen and nitrogen gas and ammonia. The ammonia produced can be used in the production of a range of products. Chief amongst these products are fertilisers. Ammonia can be used in the production of NPK fertilisers.
What is the Haber Process?
The Haber process (named for Fritz Haber) is an industrial process used to form ammonia from hydrogen and nitrogen. During the process, hydrogen and nitrogen gas are fed into a reactor where they are passed over multiple iron catalysts. The catalyst speeds up the reaction, allowing it to reach equilibrium faster. The forward reaction of the equilibrium produces ammonia which, when liquified, can be collected and sold.
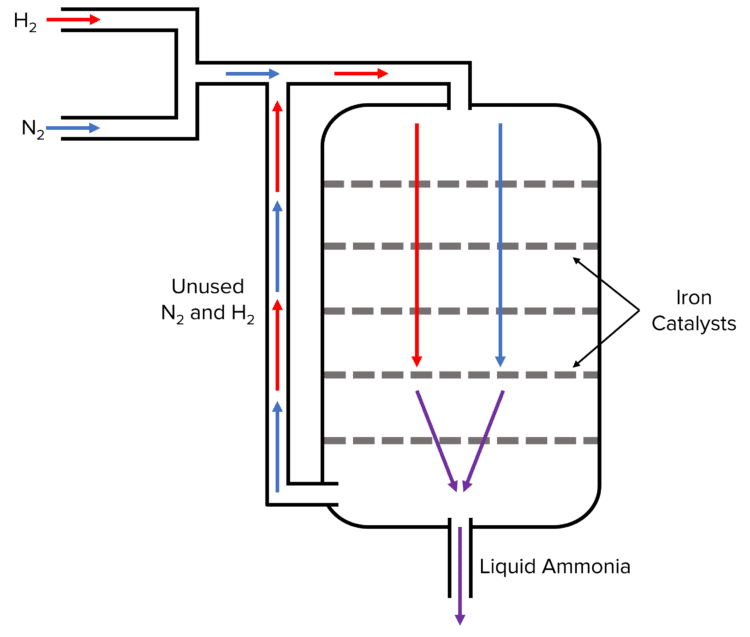
As the reaction is reversible, both hydrogen and nitrogen gas will be continually produced during this process via the reverse reaction. Instead of releasing this gas as a by product of the reaction, it is recycled back into the reactor where it will react again to form more ammonia.
The ammonia produced in the Haber process is used for a range of applications including explosives, cleaning products, and fertilisers. This process has the advantage of being relatively cheap with the raw materials (nitrogen and hydrogen) being fairly abundant. Nitrogen accounts for 78\% of the air and is easily extracted. Hydrogen can be formed by reacting steam with methane to form hydrogen and carbon dioxide.
The Conditions of the Haber Process
As the production of ammonia is a reversible reaction, we can use Le Chatelier’s principle to manipulate the reaction and produce the maximum amount of ammonia. The following equilibrium is established in the reactor:
\text{N}_2+3\text{H}_2 \rightleftharpoons 2\text{NH}_3
This reaction is exothermic in the forward direction, and produces 2 moles of gas on the right versus 4 on the left. According to Le Chatelier’s principle, an equilibrium will shift its position to oppose any changes to its conditions. As such, by decreasing the temperature of the reaction we can shift the equilibrium in the exothermic direction. This leads to a greater yield of ammonia.
However, lowering the temperature of the reaction too much will reduce the rate. This lowers the yield of ammonia. To maintain a good ammonia yield, a compromise temperature is chosen. The temperature used – around 450\degree \text{C} – is chosen to give the best yield possible.
In a similar way, a compromise pressure is used in the reaction. The equilibrium will be shifted to the forward reaction by increasing the pressure. This is the side of the reaction that has the fewest moles of gas and so is the lowest pressure. This shift increases the yield of ammonia. However, high pressures are very expensive to maintain, reducing the economic viability of the process. The pressure used (around 200\text{ atm}) is chosen as the cheapest pressure to maintain whilst still getting a good yield of ammonia.
NPK Fertilisers
One common use for the ammonia produced by the Haber process is in the manufacture of fertilisers. Fertilisers are chemical mixtures that are spread onto fields to encourage the growth of crops. Th most effective fertiliser belong to the NPK family. These are fertiliser that contain nitrogen (N), phosphorous (P), and potassium (K). These elements are incredibly useful to plant growth and can greatly improve agricultural productivity.
NPK fertilisers are produced industrially by chaining together other industrial methods. Ammonium salts, such as ammonium nitrate as well as nitric acid are produced by ammonia manufactured in the Haber process. Phosphate rock, along with potassium chloride and potassium sulphate are produced through mining operations.
The salts potassium chloride and potassium sulphate can be used directly in fertiliser manufacture, but phosphate rock cannot. It is instead treated with nitric acid to produce a soluble salt which is then added to the fertiliser.
The Haber Process Example Questions
Question 1: What is an NPK fertiliser?
[1 mark]
A fertiliser that contains nitrogen, phosphorous, and potassium.
Question 2: Explain why a temperature of 450\degree \text{C} is used in the Harber process.
[3 marks]
- The forward reaction in the Haber process is exothermic so a low temperature will increase the yield of ammonia.
- However a low temperature reduces the rate.
- A compromise temperature of 450\degree \text{C} gives the best yield at a reasonable rate.
Question 3: How does the Haber process avoid wasting hydrogen and nitrogen?
[1 mark]
By feeding (recycling) the unused gasses back into the reaction.
The Haber Process Worksheet and Example Questions
Haber Process Questions
GCSEOfficial MME
MME Premium Membership
£19.99
/monthLearn an entire GCSE course for maths, English and science on the most comprehensive online learning platform. With revision explainer videos & notes, practice questions, topic tests and full mock exams for each topic on every course, it’s easy to Learn and Revise with the MME Learning Portal.
Sign Up Now

