Using Materials
Using Materials Revision
Using Materials
In the modern world we use a range of different materials. These materials are often not pure elements but instead mixtures of different things. Mixtures of metals are called alloys, mixtures of other compounds are known as composites. Many of the metals and alloys we use are vulnerable to corrosion. This should be avoided where possible; this is usually achieved through coatings.
Alloys
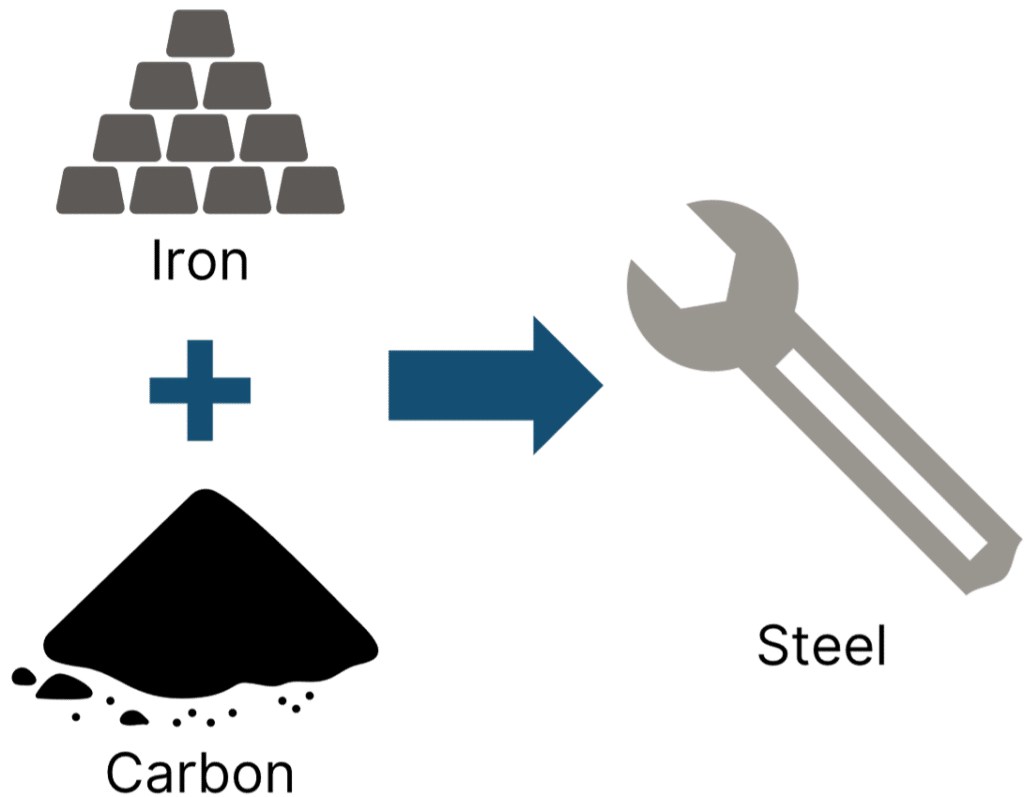
Alloys are mixtures of metals and other elements, both metal and non-metal, formulated with specific properties in mind. The majority of the metals that are used today are alloys. Perhaps the most famous alloy is steel. Used in construction, to produce tools, and in the manufacture of vehicles like ships and cars, steel is an alloy of carbon and iron.
To produce steel, specific amounts of carbon are mixed in with iron and occasionally other metals. The proportion of carbon added to the alloy will depend on the desired properties of the resulting steel. Low carbon steel is softer and more easily shaped, it is used primarily in construction and vehicle manufacture.
High carbon steel is harder but more brittle (meaning it is not easily bent or shaped) and is primarily used in the manufacture of machine parts.
Alloys are usually created to produce stronger metals than purely elemental ones. They can also produce other desirable qualities, for example, alloys of aluminium are often manufactured that have a lower density than pure aluminium. These alloys are used in the manufacture of aircraft.

Alloys are also used frequently in jewellery and art. Gold is often alloyed with other metals like silver, copper, and zinc, increasing its hardness.
The purity of gold is measured in carats. One carat is equal to roughly 5\text{ mg} gold content, with 24 carat gold equalling 100\% gold content. Gold has been used throughout history both as decorations (like gilded furniture) and as jewellery (like rings, earing, and necklaces).
Brass and bronze are also alloys that find frequent use in the arts. Both metals are alloys of copper, with bronze mixing copper with tin and brass mixing copper and zinc.
Bronze is harder than pure copper and has typically been used in sculpture and construction. Brass is more malleable than copper and is typically used for decoration (e.g. brass door handles or plaques) but has also been used in the manufacture of musical instruments.
Ceramics and Composites
Both ceramics and composites are types of materials that have been used for thousands of years. Composites are mixtures of solid materials that will have differing properties from their component materials. Ceramics are solids with high melting points that are not composed of carbon compounds.
Two common examples of ceramics are glass and clay. Glass ceramics can be formed in a number of ways. Most of glass we use day-to-day is a a ceramic known as soda-lime glass, mixture of sand, sodium carbonate, and limestone.
This suffices for most everyday uses, such as drinking glasses, mirrors, or windows, but is not always ideal for high temperature applications.
For glass that is exposed to high temperatures, borosilicate glass is used. This is glass produced from mixing sand and boron trioxide and has a higher melting point than soda-lime glass. To produce both types of glass, the components are heated to a very high temperature and allowed to melt together.
Clay ceramics such as bricks or pottery are formed shaping wet clay into desired forms and then heating to high temperatures in a kiln.
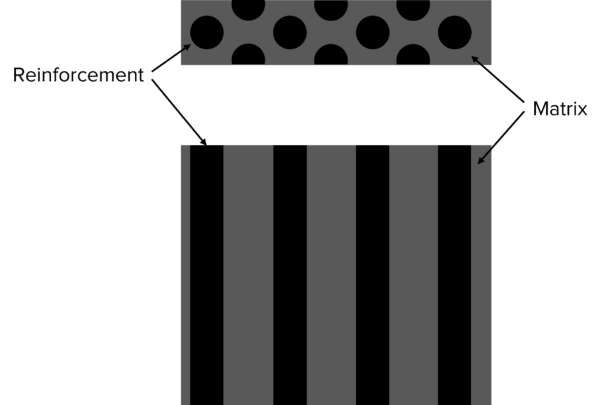
Composites are combinations of two or more materials. One material is used a matrix or binder, surrounding fibers of the other. These fibers are known as the reinforcement and are added to make the composite stronger.
Reinforced concrete is an example of a composite material used in the construction industry. To produce reinforced concrete, cement is poured around rods of steel or iron. These rods strengthen the concrete, whilst the cement allows it to remain flexible.
Polymers
Polymers are one of the most wide spread materials used by humanity. They can be produced to have a vast range of properties, from soft and malleable like the polymers used in plastic bags, to hard and brittle like the polymers used in things like window fittings.
The properties of the polymer will depend on both the monomers from which they are produced and the conditions under which they are produced. For example poly(ethene) will have different properties depending on it’s manufacture:
- Low density poly(ethene) is produced at a moderate temperature and a high pressure. Low density poly(ethene) is flexible and is used commonly for plastic bags and bottles.
- High density poly(ethne) is produced at low temperature and pressure, and employs a catalyst in its production. This yields a more rigid polymer that is used for things like water tanks and drain pipes.
Polymers can also be designed to have more specialist properties. For example thermosetting and thermosoftening polymers:
- Thermosetting polymers are ones that do not soften when heated. They contain monomers that connect polymer chains which makes the polymer solid more rigid. These connections are known as cross linked.
- Thermosoftening polymers contain no cross links. These polymers are less rigid and melt upon heating.
Corrosion
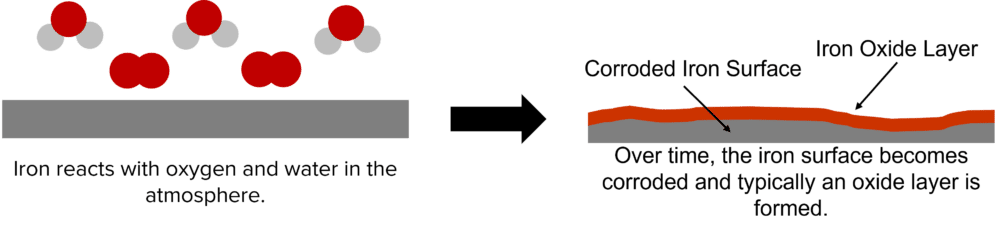
Corrosion is the process whereby metals are broken down by chemicals in the environment. Rusting is the well known form of corrosion and is the breaking down of iron by oxygen and water in the air. This process destroys the iron metal, forming a layer of iron oxide (rust) on the surface of the metal.
Corrosion is an issue when metals are exposed to the elements, reducing their structural integrity and affecting their properties. As such it, is desirable to try to prevent it. This can be done by coating the metals with a protective layer that will stop reactions between the metal and the environment.
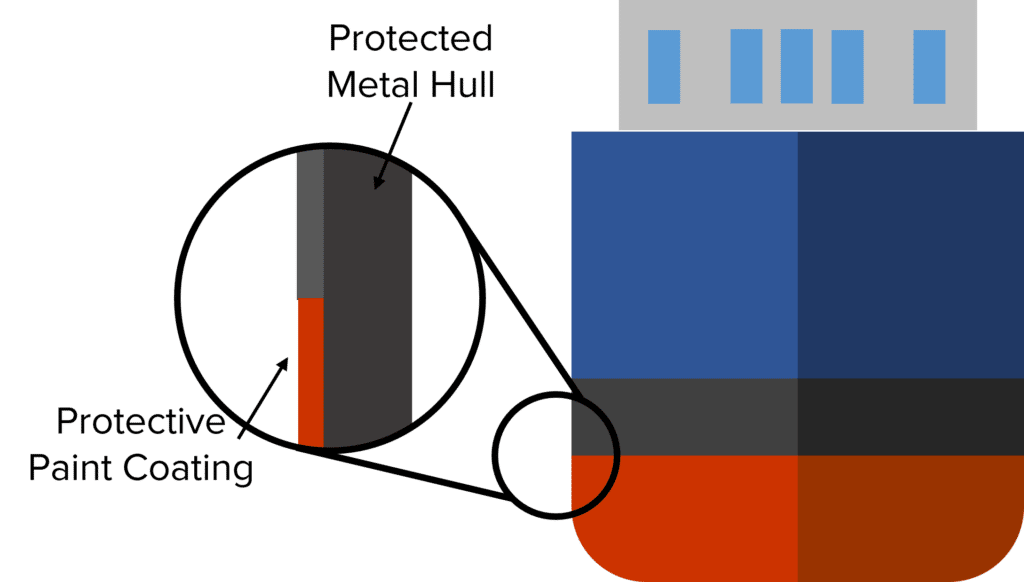
For example, the hulls of ships are at risk from corrosion by sea water. To prevent the metal hull from corroding, risking its structural integrity and allowing water to penetrate, ships are often painted to provide a protective barrier on the surface of the hull. This protective layer stops the sea water from reaching the surface of the metal preventing chemical reactions and stops the hull from being corroded.
Other forms of corrosion prevention include electroplating or greasing. In special cases, the metal may provide its own protective coating.
The oxide layer that forms when aluminium corrodes – aluminium oxide – is inert. This means it does not react further, preventing any further corrosion of the aluminium.
In some cases, a metal may be coated with another, more reactive metal. Iron can be galvanized by coating it with zinc. The zinc will react in preference to iron. The zinc will therefore corrode before the iron, providing sacrificial protection.
Using Materials Example Questions
Question 1: What is meant by the term alloy?
[1 mark]
A mixture of a metal and another element.
(Allow another metal.)
Question 2: Explain the purpose of the fibers in a composite.
[1 mark]
To strengthen the material.
Question 3: Define the term ‘thermosetting polymer’.
[1 mark]
A polymer that does not soften when heated.
Question 4: Explain the purpose of coating iron with zinc.
[3 marks]
- Zinc is more reactive than iron.
- It will therefore corrode before iron.
- This will protect the iron, increasing its lifetime.







