Reactions of Ions in Aqueous Solutions
Reactions of Ions in Aqueous Solutions Revision
Reactions of Ions in Aqueous Solution
In water, metal ions become hydrated by forming co-ordinate bonds with \text{H}_2\text{O} ligands. The metal-aqua ions of aluminium, copper, iron(II) and iron(III) form coloured precipitates when they undergo different reactions. These precipitates are used to identify the metal-aqua ion that is present in a solution.
Metal-Aqua Ions
Metal aqua-ions are complexes that have a central metal ion surrounded by water ligands. They are usually formed by dissolving transition metal salts in an aqueous solution (water). Examples of metal aqua ions include \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} and \left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+}.
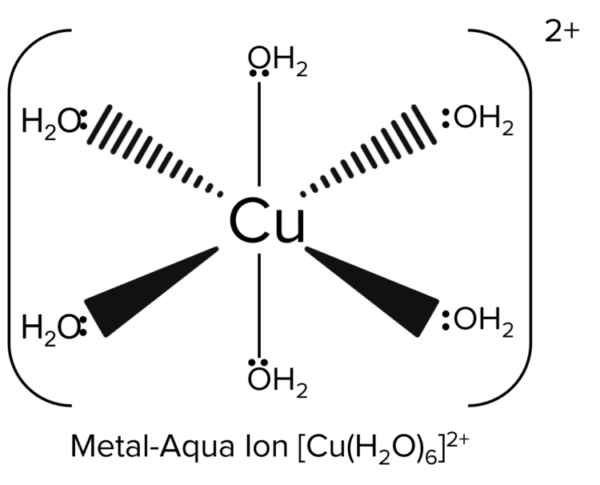
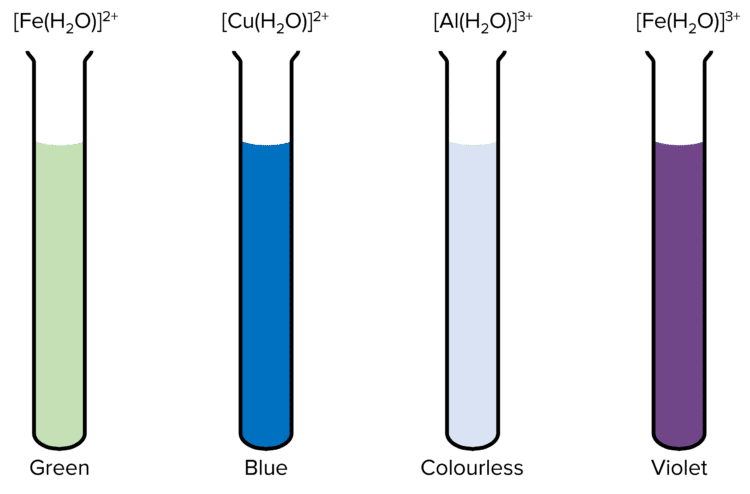
\left[\text{M}\left(\text{H}_2\text{O}\right)_6\right]^{2+} can be formed when \text{M} = \text{Fe(II)} or \text{Cu(II)}. \left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{2+} is green and \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} is blue.
\left[\text{M}\left(\text{H}_2\text{O}\right)_6\right]^{3+} can also be formed when \text{M} = \text{Al (III)} or \text{Fe (III)}. \left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} is colourless and \left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{3+} is violet.
Acidity
When we talk about the acidity of solutions we usually refer to the transfer of protons, however, it is also possible to describe acidity and basicity in terms of electrons. If a species accepts a lone pair of electrons, it is classed as a Lewis acid. If a species loses a pair of electrons, it can be classified as a Lewis base.
- Lewis Acid: Electron pair acceptor.
- Lewis Base: Electron pair donor.
When transition metals form complexes, ligands act as Lewis bases by donating a pair of electrons to the central metal ion while the central metal ion acts as a Lewis acid by accepting the lone pair of electrons.
The acidity of a metal-aqua ion will depend on the charge of the central metal ion. Iron(III) and aluminium form 3+ ions which form more acidic solutions than 2+ ions formed from iron(II) and copper (II). 3+ ions behave as stronger acids because of their higher charge density. Their high charge density means that they have greater polarising power and can therefore more easily release an H+ ions.
Reactions with Limited \text{OH}^{-} and \text{NH}_3
Metal-aqua ions can undergo reaction with limited \text{OH}^- and \text{NH}_3 to form hydroxide precipitates. In these reactions, \text{OH}^- and \text{NH}_3 act as Brønsted-Lowry bases by accepting protons from the complex ion.
In limited \text{OH}^-, the different metal aqua ions undergo the following reactions. In these reactions, \text{OH}^- accepts protons from the \text{H}_2\text{O} ligands.
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 2\text{OH}^- \rarr \text{Fe}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 + 2\text{H}_2\text{O}
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 2\text{OH}^- \rarr \text{Cu}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 + 2\text{H}_2\text{O}
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 2\text{OH}^- \rarr \text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3+ 3\text{H}_2\text{O}
\left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 2\text{OH}^- \rarr \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3+ 3\text{H}_2\text{O}
In limited \text{NH}_3 the different metal aqua ions undergo very similar reactions to when they react with \text{OH}^- ions. However, in limited \text{NH}_3, the ammonia ion accepts protons from the \text{H}_2\text{O} ligands.
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 2\text{NH}_3 \rarr \text{Fe}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 + 2\text{NH}_4^+
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 2\text{NH}_3 \rarr \text{Cu}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 + 2\text{NH}_4^+
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 2\text{NH}_3 \rarr \text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{NH}_4^+
\left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 2\text{NH}_3 \rarr \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{NH}_4^+Each metal-aqua forms a different coloured hydroxide precipitate (ppt) when it undergoes these reactions. These colours are used to identify which metal-aqua ion was involved in the reaction.
\text{Fe}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 = Green ppt
\text{Cu}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2 = Blue ppt
\text{Fe}\left(\text{H}_3\text{O}\right)_3\left(\text{OH}\right)_2 = Brown ppt
\text{Al}\left(\text{H}_3\text{O}\right)_3\left(\text{OH}\right)_2 = White ppt
| Metal-Aqua Ion | Reaction with Limited \text{OH}^{-}/\text{NH}_3 |
| \left[\text{Fe}\left(\text{H}_2\text{O}\right)\right]^{2+} | \textcolor{#c5e0b4}{\text{Fe}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2} |
| \left[\text{Cu}\left(\text{H}_2\text{O}\right)\right]^{2+} | \textcolor{#0070c0}{\text{Cu}\left(\text{H}_2\text{O}\right)_4\left(\text{OH}\right)_2} |
| \left[\text{Al}\left(\text{H}_2\text{O}\right)\right]^{3+} | \textcolor{#6b3318}{\text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3} |
| \left[\text{Fe}\left(\text{H}_2\text{O}\right)\right]^{3+} | \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 |
Reactions with Excess \text{NH}_3 and \text{OH}^-
Some metal-aqua ions undergo further reactions when excess \text{NH}_3 or \text{OH}^- is added to the solution.
Excess \underline{\text{OH}^-}
In excess sodium hydroxide \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 dissolves and becomes \left[\text{Al}\left(\text{OH}\right)_4\right]^-. In this reaction, the \text{OH}^- acts as a Brønsted-Lowry base by accepting the proton from the water ligands. This is shown in the following reaction.
\text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + \text{OH}^- \rarr \left[\text{Al}\left(\text{OH}\right)_4\right]^- + 3\text{H}_2\text{O}
Excess \underline{\text{NH}_3}
Copper undergoes an incomplete ligand substitution reaction with excess ammonia \left(\text{NH}_3\right). There are different theories as to what causes this incomplete ligand substitution to take place but the main thing you need to know is that when this reaction takes place, the precipitate dissolves to form a deep blue solution.
In this reaction, ammonia acts as a Lewis base because it donates its electron pair to the central metal ion. This can be shown through either of the following equations.
\text{Cu}\left(\text{OH}\right)_2\left(\text{H}_2\text{O}\right)_4 + 4\text{NH}_3 \rarr \left[\text{Cu}\left(\text{NH}_3\right)_4\left(\text{H}_2\text{O}\right)_2\right]^{2+} + 2\text{H}_2\text{O} + 2\text{OH}^-
OR
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{NH}_3 \rarr \left[\text{Cu}\left(\text{NH}_3\right)_4\left(\text{H}_2\text{O}\right)_2\right]^{2+} + 4\text{H}_2\text{O}
Amphoteric Salts
Amphoteric salts are salts that can act as both an acid or a base. The aluminium salt, \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3, shows amphoteric character because it reacts and dissolves in both acids and bases.
The reaction that takes place when \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 reacts with excess \text{NaOH} is an example of how the salt reacts with bases. In acids, \text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 undergoes the following reaction:
\text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{H}^+ \rarr \left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+}
Reactions in Carbonate Solutions
In carbonate solutions, metal-aqua ions act as acids. The reaction that a metal-aqua ion will go through will depend upon the charge of the central metal ion. Since metal-aqua with a \text{2+} charge are weaker acids than those with a \text{3+} charge they undergo a different reaction.
\text{2+} ions react in a carbonate solution to form insoluble carbonates and water.
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + \text{CO}_3^{2-} \rarr \text{FeCO}_3 + 6\text{H}_2\text{O}
\text{FeCO}_3 will be a green precipitate.
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + \text{CO}_3^{2-} \rarr \text{CuCO}_3 + 6\text{H}_2\text{O}
\text{CuCO}_3 will be a blue/green precipitate.
As 3+ metal-aqua ions are stronger acids than 2+ ions, they react with carbonates to form water, carbon dioxide, and a salt. In this reaction, the production of carbon dioxide gas can lead to bubbles within the solution.
2\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 3\text{CO}_3^{2-} \rarr 2\text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{CO}_2 + 3\text{H}_2\text{O}
\text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 forms a brown precipitate.
2\left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 3\text{CO}_3^{2-} \rarr 2\text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{CO}_2 + 3\text{H}_2\text{O}
\text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 forms a white precipitate.
Reactions of Ions in Aqueous Solutions Example Questions
Question 1: Sodium carbonate solution is added to a solution containing \left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} ions.
a.) Write the equation for the reaction that takes place.
b.) State two observations that may be seen when this reaction takes place.
[3 marks]
a.)
2\left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 3\text{CO}_3^{2-} \rarr 2\text{Al}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3 + 3\text{CO}_2 + 3\text{H}_2\text{O}
b.)White precipitate
Bubbles(/ effervescence/ gas given off)
Question 2: An aqueous solution reacts with limited \text{NH}_3 to form a brown precipitate. Give the formula of the metal-aqua ion involved in this reaction.
[1 mark]
\text{Fe}\left(\text{H}_2\text{O}\right)_3\left(\text{OH}\right)_3
Question 3: In a reaction, \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} reacts with excess ammonia. In this reaction ammonia acts as a Lewis base rather than a Brønsted-Lowry base. Write an equation for the reaction and state the observation you would make.
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{NH}_3 \rarr \left[\text{Cu}\left(\text{NH}_3\right)_4\left(\text{H}_2\text{O}\right)_2\right]^{2+} + 4\text{H}_2\text{O}
The solution would become deep blue.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.