Transition Metal Complexes
Transition Metal Complexes Revision
Transition Metal Complexes
Transition metals can form a range of complexes with different shapes such as octahedral or tetrahedral depending on the ligands they are bonded to. In addition to this, transition metals can also form coloured ions. The colours of which also depend on the ligands bonded as well as some other factors.
Shapes of Complex Ions
Complex ions are formed from a central metal ion and ligands. Each complex ion has a shape depending on the ligands that are bonded to the central metal ion. For example, a complex with a copper central metal ion can form both an octahedral and a tetrahedral shape.
However, an octahedral shape can only be formed with smaller ligands such as ammonia and water, meanwhile the tetrahedral shape is usually formed when larger ligands such as chloride ions are bonded.
Octahedral Complexes
When the central metal ion has six coordinate bonds, the complex will have an octahedral shape. Octahedral complexes usually form when transition metal ions are attached to small ligands such as water and ammonia.
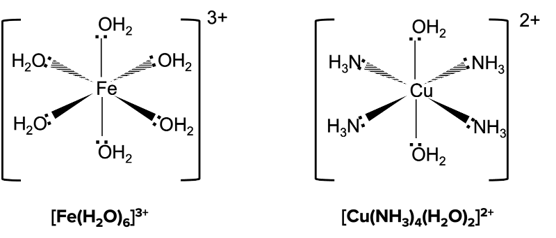
Tetrahedral Complexes
When the central metal ion has four coordinate bonds the complex will usually have a tetrahedral shape. Tetrahedral complexes usually form when transition metal ions are attached to larger ligands such as \text{Cl}^-.
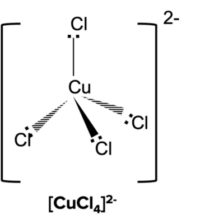
Square Planar Complexes
When the central metal ion has Four coordinate bonds the complex could also have a square planar shape.
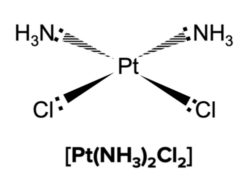
Linear Complexes
When the central metal ion has two coordinate bonds, the complex will have a linear shape. Silver most commonly forms linear complexes
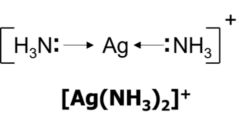
Cis-Trans and Optical Isomerism
Complex ions can show different types of isomerism, specifically cis-trans isomerism or optical isomerism.
Cis-Trans Isomerism
Cis-trans isomerism is a special case of E-Z isomerism. Both octahedral and square planar complexes can display cis-trans isomerism.
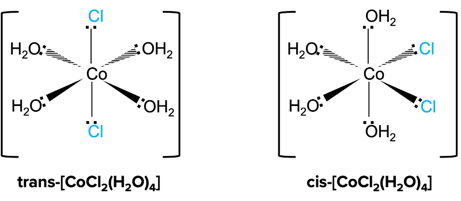
An octahedral complex shows cis-trans isomerism when two of the monodentate ligands are the same, while the other four monodentate ligands are different.
Similar to E-Z isomerism, trans isomers have two identical ligands on opposite sides while cis isomers have two identical ligands on the same side of the central metal ion.
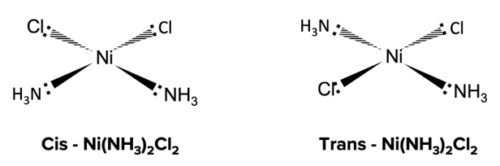
Square planar complexes show cis-trans isomerism when two of the monodentate ligands are the same, while the other two monodentate ligands are different.
Optical Isomerism
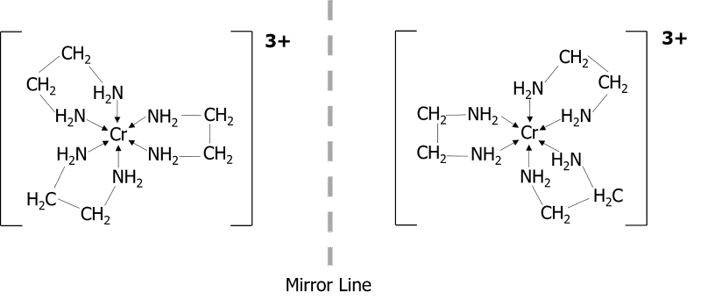
Octahedral complexes display optical isomerism when there are 3 bidentate ligands such as ethane-1,2-diamine \left(\text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2\right) attached to the central metal ion. The isomers are mirror images which are non-superimposable.
Cisplatin
Cisplatin is the cis-isomer of a platinum complex that is commonly used as a treatment for cancer. It consists of ammonia and chloride ligands, with either of the chlorine ligands undergoing ligand substitution in order to treat cancer.

When treating cancer, the chlorine ligand is usually substituted with a nitrogen atom which is located on the DNA of a cancer cell. This substitution causes the shape of the DNA to change and prevents DNA replication in cancer cells.
Taking Cisplatin has quite a few side effects. Cisplatin cannot differentiate between cancer cells and some types of healthy cells. Kidney damage and hair loss are some side effects caused by Cisplatin. Due to this, only small quantities of Cisplatin are used so that the side effects are reduced.
Formation of Coloured Ions
Transition metal ions can be identified using their colours. The colour of transition metal ions are determined by their d-orbital splitting. This in turn is determined by three factors: the oxidation state, coordination number, and ligand type.
Oxidation state
In the example below, the charge on the central metal ion, \text{Co}^{3+}, changes which subsequently changes the oxidation state of the complex and the colour of the complex ion.
\left[\text{Co}\left(\text{NH}_3\right)_6\right]^{2+} \rarr \left[\text{Co}\left(\text{NH}_3\right)_6\right]^{3+} + \text{e}^-
Ligand
In the example below, all the ligands change from \text{H}_2\text{O} to \text{NH}_3 which causes a change in the colour of the complex ion.
\left[\text{Co}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 6\text{NH}_3 \rarr \left[\text{Co}\left(\text{NH}_3\right)_6\right]^{2+} + 6\text{H}_2\text{O}
Coordination number
In the example below, the number of ligands bonded to the central metal ion changes from 6 to 4 which causes a change in the colour of the complex.
\left[\text{Co}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{Cl}^- \rarr \left[\text{Co}\text{Cl}_4\right]^{2-} + 6\text{H}_2\text{O}
Colours arise when electrons in the d-subshell transition from a ground state to an excited state. This happens because some of the wavelengths of visible light are absorbed causing the d-electrons to move to higher energy levels. The remaining light is transmitted and gives the substance colour.
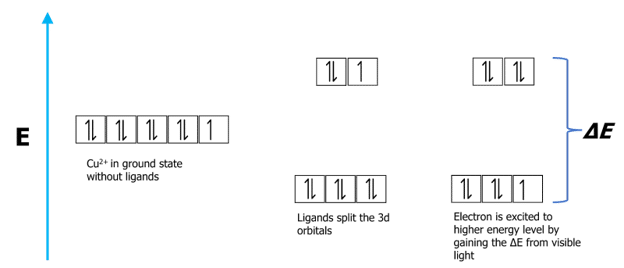
The energy difference between the ground state and the excited state of d electrons (known as d-splitting) can be calculated using the following equation.
\Delta{E}=h\nu=\frac{hc}{\lambda}
\Delta\text{E} is for energy difference between split orbitals in Joules \text{(J)}
h is for Planck’s constant = 6.63\times10^{-34}\text{ J s}
\nu is for frequency in Hertz \text{(Hz)}
c is for the speed of light = 3.00\times10^{8}\text{ m s}^{-1}
\lambda is for wavelength of light in metres \text{(m)}
For colourless compounds, there are either no electrons in 3d orbitals \left(\text{Sc}^{3+}\right) or all 3d orbitals are full \left(\text{Zn}^{2+}, \text{Cu}^+\right) so electrons cannot be excited.
Colourimetry
Colorimetry can be used to determine the concentration of a coloured complex ion. In colorimetry, a source of white light passes through a filter to produce a light of only one colour.
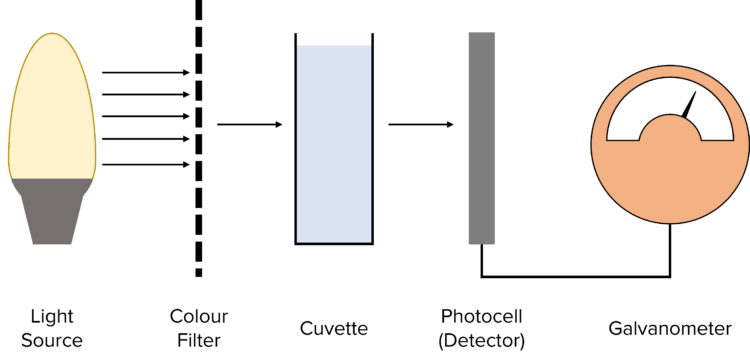
Steps to determine the concentration of a solution of copper (II) ions with colorimetry:
- Make up solutions of known concentration.
- Shine white light through a filter which only lets the colour absorbed by the solution through.
- Measure the amount of light that was absorbed/ transmitted with a colorimeter.
- Plot graph of relative absorption vs concentration (calibration curve). You should find that the amount of light absorbed is proportional to the concentration of the absorbing species.
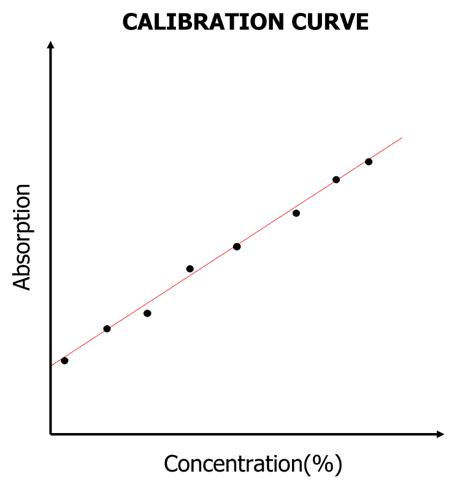
Measure the absorbance of the copper (II) solution and find the concentration with the calibration curve.
When carrying out colorimetry measurements, there are a few things that you need to make sure of as they could significantly affect the results.
The cuvette used for each sample should have the same dimensions since absorption is dependent on distance travelled through a solution.
The coloured filter used has to complement the colour most strongly absorbed by the sample.
Transition Metal Complexes Example Questions
Question 1: Explain why the complex ion formed in an aqueous solution between copper (II) ions and chloride ions is a different colour from the \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+}.
[3 marks]
- In different complexes the d splitting will be different.
- Light energy is absorbed which causes an electron to be excited.
- Different frequency/ wavelength of light is absorbed/ transmitted
Question 2: Explain why metal (II) ions would not usually form octahedral complexes if all the ligands were chloride ions.
[1 mark]
The chloride ligand is large (so generally forms tetrahedral complexes).
Question 3: \left[\text{Pd}\left(\text{NH}_3\right)_4\right]\text{SO}_4 is an ionic compound. Name two possible shapes for the complex palladium ion in this compound.
[2 marks]
- Square planar
- Tetrahedra
Question 4:
\Delta\text{E}=\text{h}\nu is the equation that can be used to determine the frequency of light absorbed by a complex ion.
a.) State the meaning of the symbols \Delta\text{E} and h.
b.) Give three factors which result in a change in the frequency of light absorbed.
[5 marks]
a.)
\Delta\text{E}: energy absorbed by electron.
h: Planck’s Constant.
b.)
- Change in oxidation state.
- Change in ligand.
- Change in coordination number.
Question 5: Colorimetry is a method used to determine the concentration of a coloured complex ion.
a.) In colorimetry, the cuvette used for each sample has to have the same dimensions. Explain why.
b.) Suggest why coloured filters are used in colorimetry.
c.) Suggest one reason why a colorimetric method might be done instead of a titration.
[3 marks]
a.) Absorption is proportional to distance travelled through the solution.
b.) To select the colour(/ wavelength/ frequency) that is most strongly absorbed by the sample OR the filter colour complements the colour of the solution.
c.) It is quicker to analyse samples OR colorimetry uses smaller volumes of solution.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.