Transition Metals and Substitution Reactions
Transition Metals and Substitution Reactions Revision
Transition Metals and Substitution Reactions
Transition metals are elements in the d-block of the periodic table that can form at least one ion with an incomplete d orbital. The incomplete d orbital allows for transition metals to have a variety of properties such as forming complex ions that can undergo substitution reactions.
Transition Metal Elements
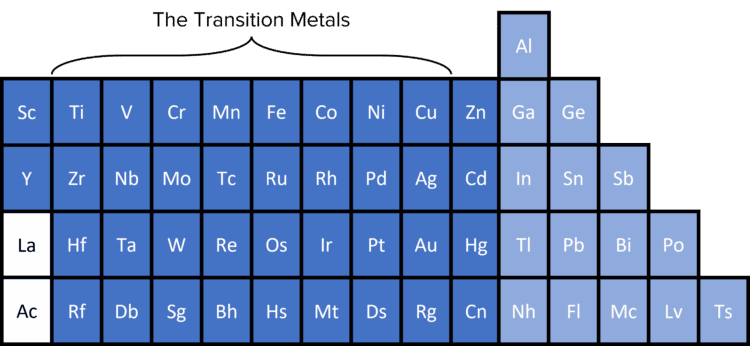
You are most likely more familiar with transition metals being the elements in the centre of the periodic table or, in other words, the d-block elements.
However, transition metals are actually defined as elements that can form one or more stable ions with a partially filled d sub-level. For example, iron \left(\text{Fe}\right) can lose electrons to form an iron ion \left(\text{Fe}^{3+}\right) with an incomplete sub-shell and is therefore considered a transition metal.
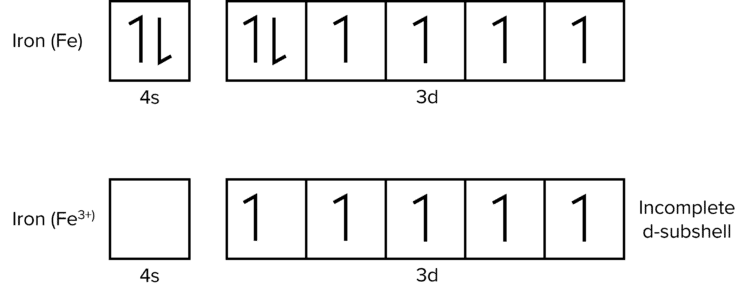
The Odd One’s Out
Within the period 4 d-block, two of the metals are not classed as transition metals, zinc and scandium.
While zinc is in the d block of the periodic table, it can only form the \text{Zn}^{2+} ion, which has a full d sub-level. As the \text{Zn}^{2+} ion has a full d sub-level, zinc is not classed as a transition metal.
Scandium is also not classed as a transition metal because it does not have a partially filled d sub-shell. The scandium ion \left(\text{Sc}^{3+}\right) has no electrons in its d sub-shell and therefore cannot be classed as a transition metal.
Transition Metal Properties
Transition metals have a range of chemical properties because of their ability to form incomplete d sub-shells.
The four main characteristic properties of transition metals are:
- Complex Formation: Transition metals can undergo reactions with other molecules/ions to form complexes where the transition element is central and other molecules/ions surround it.
- Formation of Coloured Ions: Due to the incomplete d sub-shells in transition elements, energy can be absorbed or transmitted to produce a colour.
- Variable Oxidation States: Transition elements are able to form multiple ions each with a different oxidation state.
- Catalytic Activity: Some transition metals are able to act as catalysts for different reactions.
Complex Ions
One of the main characteristics of transition metals is that they are able to form complexes. Complex ions usually consist of a central metal ion which is surrounded by ligands.
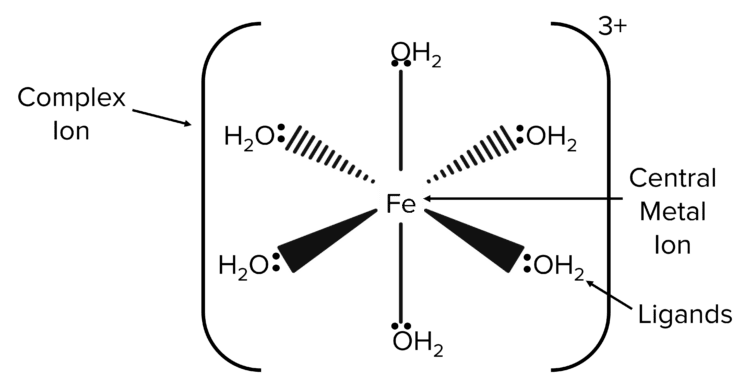
Central metal ion: A central metal ion is the metal ion at the centre of a complex. It is usually a transition metal and is surrounded by ligands. \left[\text{Al}\left(\text{H}_2\text{O}\right)_6\right]^{2+} is an example of a complex.
Ligand: A molecule or ion that forms a coordinate bond with a transition metal by donating a lone pair of electrons.
Ligands are usually bonded to a central metal ion by a coordinate bond. A coordinate bond is formed when the shared pair of electrons in a covalent bond comes from just one of the bonded atoms. The coordination number of a complex tells us the number of coordinate bonds to the central metal atom or ion.
Complex ions can undergo different kinds of reactions such as substitution reactions. In substitution reactions, the ligands within a complex can be substituted with different ligands. These ligands can either monodentate, bidentate, or multidentate.
Substitution Reactions: Monodentate Ligands
A monodentate ligand is one where only one atom donates an electron pair. \text{H}_2\text{O}, \text{NH}_3, \text{Cl}^{-} and \text{CN}^{-} are all examples of monodentate ligands. Monodentate ligands can be ions \left(\text{Cl}^{-}\right) or neutral molecules (\text{NH}_3).
When \text{H}_2\text{O} is replaced with \text{NH}_3 there is no change in coordination number due to the fact that they are the same size.
Some substitution reactions can be incomplete. In the formation of \left[\text{Cu}\left(\text{NH}_3\right)_4\left(\text{H}_2\text{O}\right)_2\right]^{2+}, the substitution reaction is incomplete, and the complex has a mixture of ammonia and water ligands. This is due to the higher concentration of \text{H}_2\text{O} within the solution.
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} +4\text{NH}_3 \rarr \left[\text{Cu}\left(\text{NH}_3\right)_4\left(\text{H}_2\text{O}\right)_2\right]^{2+} + 4\text{H}_2\text{O}
Chloride Ions
A ligand substitution reaction can occur when chloride ions are added to aqueous complex ions.
The \text{Cl}^- ligand is larger than the \text{H}_2\text{O} and \text{NH}_3 ligands, so the ligand exchange reaction between water and the chloride ligand results in a change in coordination number.
The following reactions show the change in coordination number when chloride ions are involved in substitution reactions.
\left[\text{Fe}\left(\text{H}_2\text{O}\right)_6\right]^{3+} + 4\text{Cl}^- \rarr \left[\text{FeCl}_4\right]^- + 6\text{H}_2\text{O}
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{Cl}^- \rarr \left[\text{CuCl}_4\right]^2- + 6\text{H}_2\text{O}
\left[\text{Co}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{Cl}^- \rarr \left[\text{CoCl}_4\right]^2- + 6\text{H}_2\text{O}
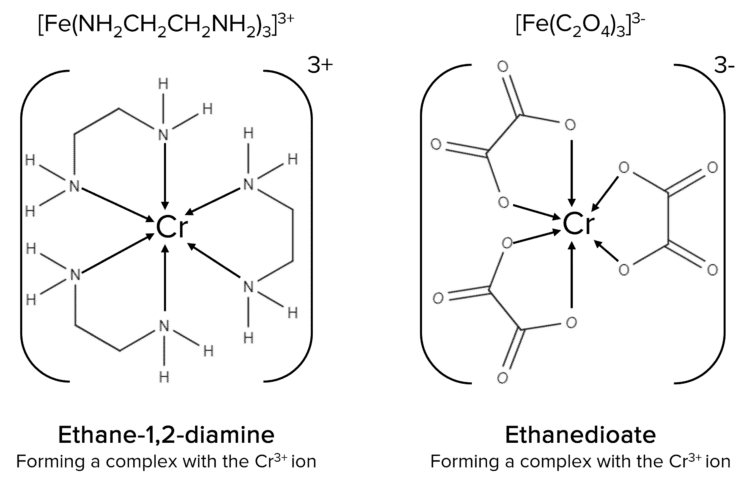
Substitution Reactions: Bidentate Ligands
Bidentate ligands can form two coordinate bonds to the central metal ion. The two bidentate ligands that you need to be aware of are ethane-1-2-diamine, \text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2, and ethanedioate, \text{C}_2\text{O}_4.
Examples
\left[\text{Cr}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 3\text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2 \rarr \left[\text{Cr}\left(\text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2\right)_3\right]^{2+} + 6\text{H}_2\text{O}
\left[\text{Cr}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 3\text{C}_2\text{O}_4^{2-} \rarr \left[\text{Cr}\left(\text{C}_2\text{O}_4\right)_3\right]^{4-} + 6\text{H}_2\text{O}
Substitution Reactions: Multidentate Ligands
Multidentate ligands can form more than two coordinate bonds to the central metal ion.
EDTA is the only multidentate ligand that you will need to know about. It is called a hexadentate ligand, as it forms six coordinate bonds with a central metal ion in a ligand substitution reaction.
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + \text{EDTA}^{4-} \rarr \left[\text{Cu}\left(\text{EDTA}\right)\right]^{2-} + 6\text{H}_2\text{O}
Haemoglobin
Haemoglobin is crucial in transporting oxygen around the body. It is an example of an important molecule in the body that needs a multidentate ligand in order to carry out its function.
Haem is part of haemoglobin. It contains an iron (II) central metal ion that forms six coordinate bonds with one multidentate ligand.
In haemoglobin, oxygen forms a coordinate bond to \text{Fe(II)} which enables oxygen to be transferred in the blood. Carbon monoxide is toxic as it forms a stronger bond to the \text{Fe(II)}, stopping oxygen from being delivered to the cells.
The Chelate Effect
When substitution reactions take place, it is more likely for bidentate and multidentate ligands to replace monodentate ligands to form complexes that are more stable. This is known as the chelate effect. The chelate effect can be explained by applying the Gibbs Free Energy equation, \Delta G = \Delta H – T\Delta S, to a substitution reaction.
The enthalpy change for a ligand substitution is small as there are a similar number of bonds in each complex. Meanwhile, the entropy change for the reaction is positive because there are more molecules of products than reactants. The small enthalpy change coupled with the positive entropy leads to a negative Gibbs free energy value meaning that the complex formed is stable.
For example, we can have a look at the substitution reaction between \left[\text{Cr}\left(\text{H}_2\text{O}\right)_6\right]^{2+} and \text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2.
\left[\text{Cr}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 3\text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2 \rarr \left[\text{Cr}\left(\text{NH}_2\text{CH}_2\text{CH}_2\text{NH}_2\right)_3\right]^{2+} + 6\text{H}_2\text{O}
In this reaction, the enthalpy change of the reaction is small as the \text{Cr}^{2+} central metal ion still forms 6 coordinate bonds. The entropy change is positive since the number of molecules changes from 4 on the left to 7 on the right. Within the Gibbs Free Energy equation, this would mean \Delta\text{G} is negative and therefore more stable.
Transition Metals and Substitution Reactions Example Questions
Question 1: In an aqueous solution, the \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} ion reacts with an excess of ethane-1,2-diamine to form the complex ion Z.
a.) Write an equation for this reaction.
b.) Explain, in terms of the chelate effect, why the complex ion Z is formed in preference to the \left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} complex ion.
[3 marks]
a.)
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 3\text{H}_2\text{NCH}_2\text{CH}_2\text{NH}_2 \rarr \left[\text{Cu}\left(\text{H}_2\text{NCH}_2\text{CH}_2\text{NH}_2\right)_3\right]^{2+} + 6\text{H}_2\text{O}
b.)
The number of particles increases from 4 to 7
So the entropy change is positive (/ disorder increases/ entropy increases).
Question 2:
a.) Give an example of a bidentate ligand.
b.) Write an equation for the substitution reaction, involving copper, where there is a change in coordination number and a change in the charge of the complex ion.
[3 marks]
a.)
\text{H}_2\text{NCH}_2\text{CH}_2\text{NH}_2 OR \text{C}_2\text{O}_4^{2-}
(Allow ligand names)
b.)
\left[\text{Cu}\left(\text{H}_2\text{O}\right)_6\right]^{2+} + 4\text{Cl}^- \rarr \left[\text{CuCl}_4\right]^{2-} + 6\text{H}_2\text{O}
(allow suitable alternatives)
Question 3: Give any two characteristics of transition metals.
[2 marks]
Any two from:
- Catalytic action.
- Variable oxidation states.
- Formation of complex ions.
- Formation of coloured ions.
Question 4: Define the terms ligand and coordination number.
[2 marks]
- Ligand: An atom, ion or molecule that can donate a lone electron pair.
- Coordination Number: The number of coordinate bonds formed to a central metal ion or number of electron pairs donated or donor atoms.
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.