Energy Stores and Systems
Energy Stores and Systems Revision
Energy Stores and Systems
Energy cannot be created or destroyed, however it can be stored or transferred in many different ways.
Energy Stores
Energy is stored in different types of energy stores. The main types of energy stores are:
Kinetic – energy stored by an object that is moving.
Magnetic – energy stored by two magnets attracting or repelling one another.
Thermal – energy stored in an object due to the heat of the object.
Chemical – energy stored by chemical bonds.
Elastic Potential – energy stored by an object that has been stretched or squashed.
Electrostatic – energy stored by two charges attracting or repelling one another.
Gravitational Potential – energy stored by an object when it is lifted to a height.
Nuclear – energy stored inside the nuclei of atoms.
Energy Transfers
Energy can be transferred between energy stores by one of the following ways:
Mechanical Work – when a force moves an object a distance.
Heating – when energy is transferred to a colder object from a hotter object.
Electrical Work – when energy is transferred by a moving charge due to a potential difference.
Radiation – when energy is transferred as an electromagnetic wave e.g. gamma-rays or UV radiation.
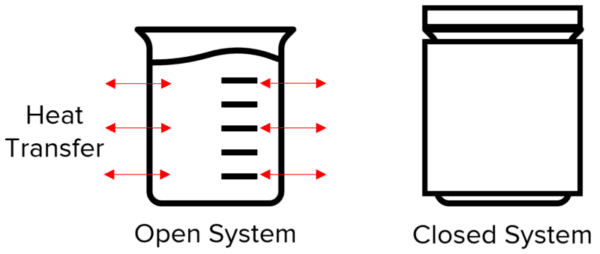
Closed and Open Systems
A system is an object or a group of objects that you are dealing with.
Whenever a system changes, energy is transferred.
If a system is closed energy can neither enter nor leave the system. This means that whenever energy is transferred in the system, the total energy remains the same, or net change =0.
If a system is open energy can be transferred into or out of the system.
For example, a beaker full of hot water is an open system because heat energy may transfer out of the beaker to the surroundings. An insulated beaker is a closed system because heat energy cannot be transferred to the surroundings.
Doing Work
The amount of work done is a different way of saying the amount of energy transferred.
e.g. When a bodybuilder lifts a weight, they do work on the weight by transferring chemical energy stored in their body to gravitational potential energy to the weight.
e.g. When you push a shopping trolley, you do work on the shopping trolley by transferring chemical energy stored in your body to kinetic energy of the trolley.
Energy Stores and Systems Example Questions
Question 1: What type of energy store is only stored by attracting or repelling charged objects?
[1 mark]
Electrostatic.
Question 2: A ball is thrown into the air. What types of energy store are transferred to the ball as it travels upwards?
[2 marks]
Kinetic and Gravitational Potential.
Kinetic energy is gained because the ball is moving.
Gravitational potential energy is gained because the ball moves to a higher position.
Question 3: When water is warming up in a kettle, what type of energy transfer is increasing the temperature of the water?
[1 mark]
Heating.
Question 4: Two balls are in a closed system. When energy is transferred between the balls, what is the net change in the energy of the system?
[1 mark]
The net energy change is zero because this is a closed system and so no energy can be transferred in or out of the system.





