Nuclear Instability
Nuclear Instability Revision
Nuclear Instability
As previously discussed, radioactive isotopes are radioactive because they have a nucleus that is unstable. The reason for giving off radiation is to improve the stability of the nucleus.
The N-Z Graph
A graph of the number of protons (Z) against the number of neutrons (N) can be used to predict which isotopes are stable and which will emit alpha or beta radiation. Below is a N – Z graph:
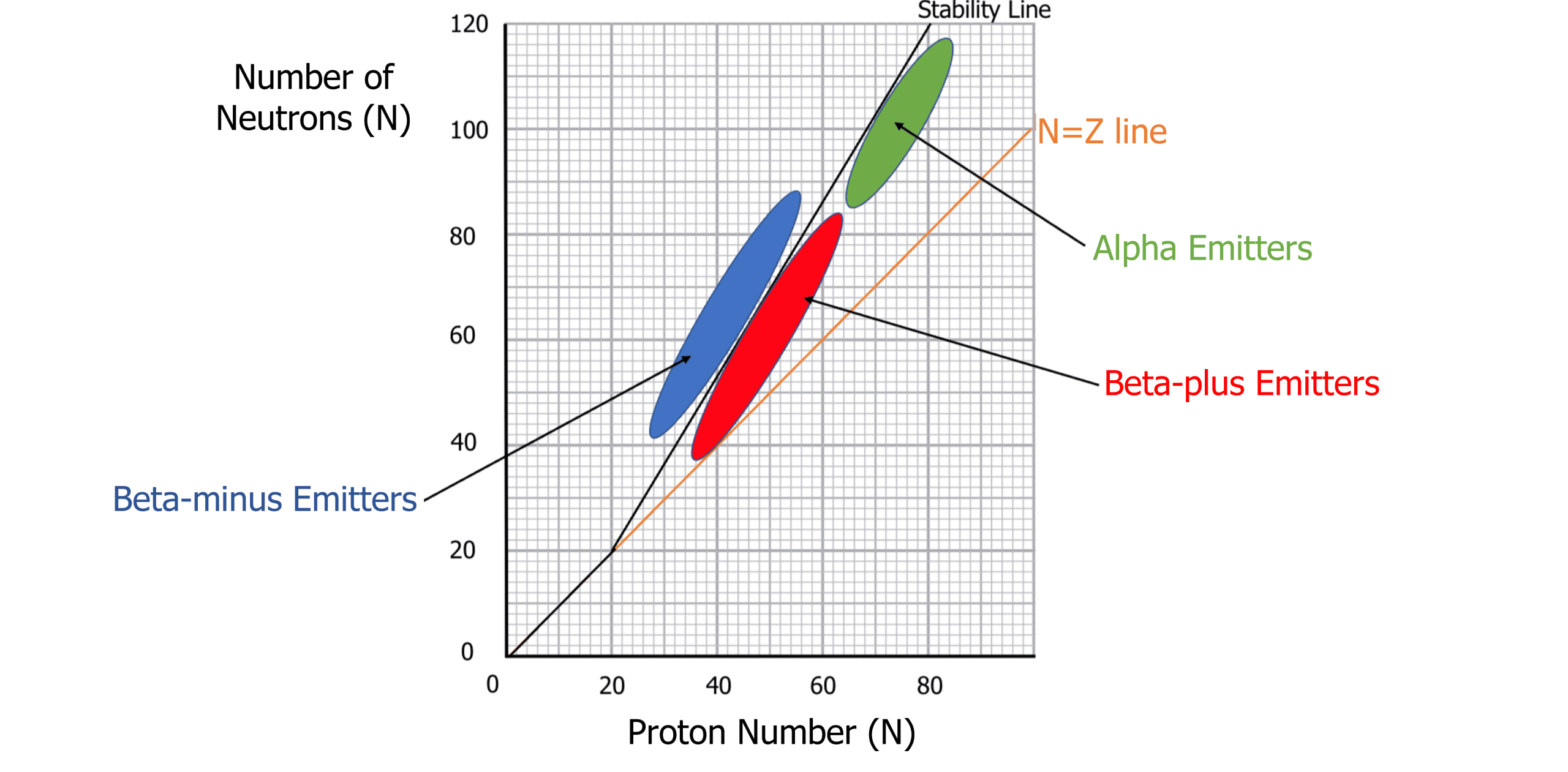
The bold black line running through the graph represents the stability line. Isotopes that fall along this line are usually stable. Most elements below a proton number of \bold{20} are generally considered stable although isotopes with too many or too few neutrons can become more unstable (such as carbon-14).
Beyond a proton number of \bold{20}, it is common for isotopes to have a greater number of neutrons than protons to maintain stability. This is because the neutrons are needed to prevent the electrostatic repulsion between neighbouring protons. The neutrons also contribute to the binding force within the nucleus.
The area highlighted in green represents where most alpha emitters are found. The nucleus emits alpha radiation to decrease the size of the nucleus as the nucleus is too large to maintain stability.
The area highlighted blue represents beta minus emitters. Beta minus emission involves a neutron becoming a proton, therefore increasing the proton number and decreasing the neutron number which brings the N-Z ratio back to the stability line.
The area represented by red is the beta plus emitters. Beta plus emission invokes a proton becoming a neutron, therefore bringing the N-Z ratio back to where it needs to be to maintain stability. Isotopes in this section may also experience the process of electron capture. In this process, the nucleus of the atom absorbs one of its own electrons. This causes a proton to become a neutron and gamma radiation emitted.
Decay Equations
A decay equation can be used to show the changes that occur due to the radioactive decay process.
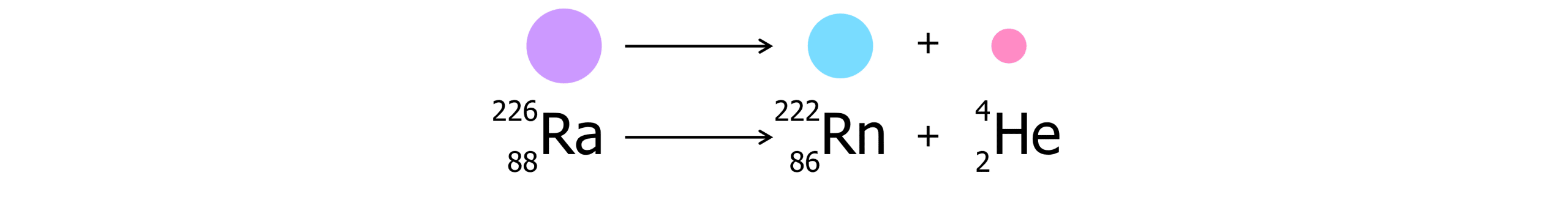
Alpha decay:
In alpha decay, an alpha particle is emitted which is comprised of 2 protons and 2 neutrons.
- Alpha particle is emitted
- The mass number of the daughter nuclei decrease by 4
- The proton number decreases by 2
- A different element is formed (because the proton number changes)
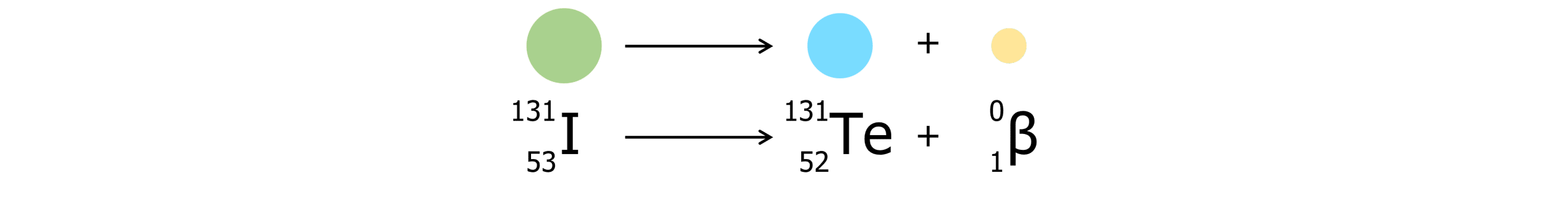
Beta plus decay:
In beta plus decay, a proton becomes a neutron and a positron is released into the surroundings.
- Mass number remains unchanged
- Proton number decreases by 1.
- A different element is formed as the number of protons have changed.
Beta minus decay:
In beta minus decay, a neutron becomes a proton and an electron is released into the surroundings.
- Mass number remains unchanged
- Proton number increases by 1.
- A different element is formed as the number of protons have changed.
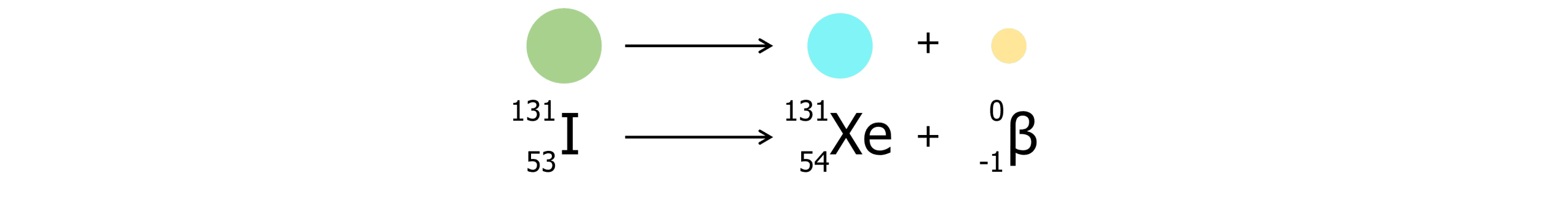
Nuclear Instability Example Questions
Question 1: Describe what a N – Z graph shows.
[3 marks]
A N – Z graph shows the number of protons against the number of neutrons for a selection of isotopes.
It can be used to predict which isotopes are stable and which are not.
Also for those that are unstable, it can be used to predict which type of radiation will be emitted.
Question 2: What makes a nucleus unstable?
[2 marks]
The nucleus has too few or too many protons.
Question 3: Write the decay equation for the alpha decay of carbon-14.
[3 marks]
1 mark for C \rightarrow Be + He
1 mark for ^{10}_4 Be
1 mark for ^4_2 He








