Molecular Kinetic Theory
Molecular Kinetic Theory Revision
Molecular Kinetic Theory Model
This section looks more closely at the link between the kinetic energy of the particles of a gas, and the pressure they exert on the walls of the container they are stored in.
Molecular Kinetic Theory Model
The molecular kinetic theory model suggests that molecules of a gas are moving around randomly with kinetic energy. They exert a pressure on the walls of the container every time they collide with them.
The molecular kinetic theory model is a theory meaning it has been derived from other theories and assumptions. This is unlike the gas laws which are based on true experimentation and evidence.
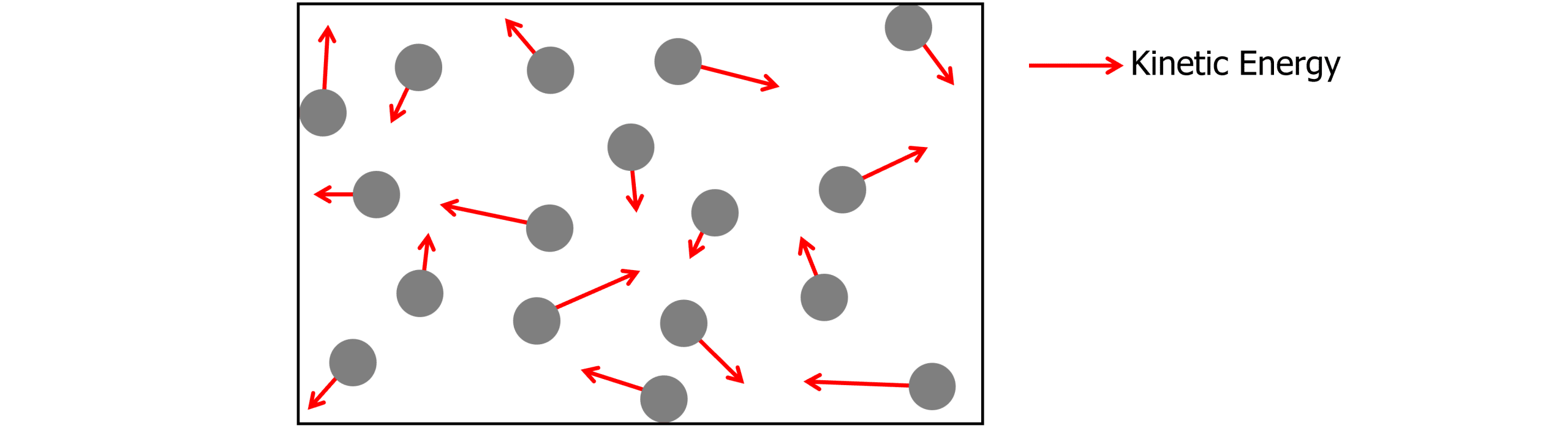
Assumptions
When using the molecular kinetic theory model, we need to make a set of assumptions. These are:
- The volume of each molecule is considered to be negligible.
- All collisions with the walls of the container are elastic.
- The gas molecules do not interact with each other (no collisions, no intermolecular forces)
- The gas obeys the three gas laws.
This theory therefore suggests that molecules only have kinetic energy and zero potential energy as there are no intermolecular forces. Therefore the internal energy of an ideal gas is its kinetic energy only.
Kinetic Theory of Gases Equation
To understand the kinetic theory of gases equation, we first need to consider the molecules of the gas. Each of the molecules will have its own kinetic energy but not all will have the same energy and therefore, may not have the same velocity. This brings the idea of the root mean squared velocity (c_{\text{rms}}).
The root mean squared velocity (c_{\text{rms}}) is an accurate way of calculating the average speed of all molecules and is the square root of the average of all particles velocity squared.
The kinetic theory of gases equation states:
PV=\dfrac{1}{3}Nm(c_{\text{rms}})^2
- P= pressure in pascals \text{(Pa)}
- V= volume in cubic metres \text{(m}^3\text{)}
- N= the number of molecules in the gas
- m= the mass of one molecule of the gas in kilograms \text{(kg)}
- c_{\text{rms}}= the root mean squared speed in metres per second \text{(ms}^{-1}\text{)}
Average Molecular Kinetic Energy
The average kinetic energy of each molecule of a gas in a container can also be calculated using the equation below:
E_k=\dfrac{1}{2}m(c_{\text{rms}})^2=\dfrac{3}{2}kT=\dfrac{3}{2}\dfrac{RT}{N_A}
- E_k= the average kinetic energy in joules \text{(J)}
- m= the mass of each gas molecule in kilograms \text{(kg)}
- k= the Boltzmann constant (=1.38 \times 10^{-23} \text{ kgm}^2\text{s}^{-2}\text{K}^{-1})
- T= the temperature in kelvin \text{(K)}
- R= the molar gas constant (=8.31 \text{ kgm}^2\text{s}^{-2}\text{K}^{-1}\text{mol}^{-1})
- N_A= Avogadro’s number (=6.02 \times 10^{23})
Example: Calculate the average kinetic energy of a molecule of hydrogen-2. The c_{\text{rms}} of hydrogen-2 is 700 \text{ ms}^{-1}.
[3 marks]
Firstly we need the mass of a single hydrogen-2 molecule:
1 \text{ mol}= 2 \text{ g} = 0.002 \text{ kg}
Therefore the mass of one molecule:
\dfrac{0.002}{6.02 \times 10^{23}} = \bold{3.32 \times 10^{-27}} \textbf{ kg}
Then substitute values into the equation for energy:
\begin{aligned} \bold{E_k} &= \bold{\dfrac{1}{2}mc_{\text{rms}}} \\ &= \dfrac{1}{2} \times 3.32 \times 10^{-27} \times \textcolor{f21cc2}{700}^2 \\ &= \bold{8.13 \times 10^{-22} \textbf{ J}} \end{aligned}
Brownian Motion
If very small particles (such as smoke or a powder) are placed into a container of gas or liquid, the particles can be observed via a microscope as moving randomly and erratically. This random motion is known as Brownian motion.
As the particles move randomly, this observation shows that particles have a range of kinetic energies and no preferred direction of motion.
As the particles of the gas or liquid are too small to be observed directly, the larger particles need to be added. These larger particles are able to be moved by the smaller particles as they have such high kinetic energies.
As the larger particles can be seen to collide with the walls of the container, this also acts as evidence for the concept of pressure.
Molecular Kinetic Theory Example Questions
Question 1: State the assumptions made when using the kinetic theory model of gases.
[4 marks]
- The volume of each molecule is considered to be negligible.
- All collisions with the walls of the container are elastic.
- The gas molecules do not interact with each other (no collisions, no intermolecular forces).
- The gas obeys the three gas laws.
Question 2: Using the molecular kinetic theory model, describe why a gas exerts a pressure on a container.
[3 marks]
The molecular kinetic theory model states that each molecule of a gas has its own kinetic energy and therefore a velocity.
As the molecule moves it eventually collides with the walls of the container causing its momentum to change.
As the momentum changes, a force is applied to the wall of the container. This force causes a pressure on the container wall.
Question 3: Explain what is meant by root mean squared speed.
[1 mark]
The root mean squared is the square root of the average velocity squared of all molecules in the container.
Question 4: Calculate the average kinetic energy of a molecule of O_2 . The c_{\text{rms}} of O_2 is 450 \text{ ms}^{-1}.
[3 marks]
Mass of O_2:
1 \text{ mol}= 32 \text{ g} = 0.032 \text{ kg}Mass of one molecule = \dfrac{0.032}{6.02 \times 10^{23}} = \bold{5.32 \times 10^{-26}} \textbf{ kg}
\begin{aligned} \bold{E_k} &= \bold{\dfrac{1}{2}mc_{\text{rms}}} \\ &= \dfrac{1}{2} \times 5.32 \times 10^{-26} \times 450^2 \\ &= \bold{5.39 \times 10^{-21}} \textbf{ J} \end{aligned}You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.