Alpha, Beta and Gamma Radiation
Alpha, Beta and Gamma Radiation Revision
Alpha, Beta and Gamma Radiation
Before we can look at radioactive decay in detail, we need to understand the three types of radiation and some of their applications.
Alpha Radiation
A single alpha particle consists of two protons and two neutrons, the same as the nucleus of a helium atom.

Some properties of alpha radiation include:
- Very ionising (because each particle has a charge of 2+)
- Affected by electric fields (attracted to negative charges)
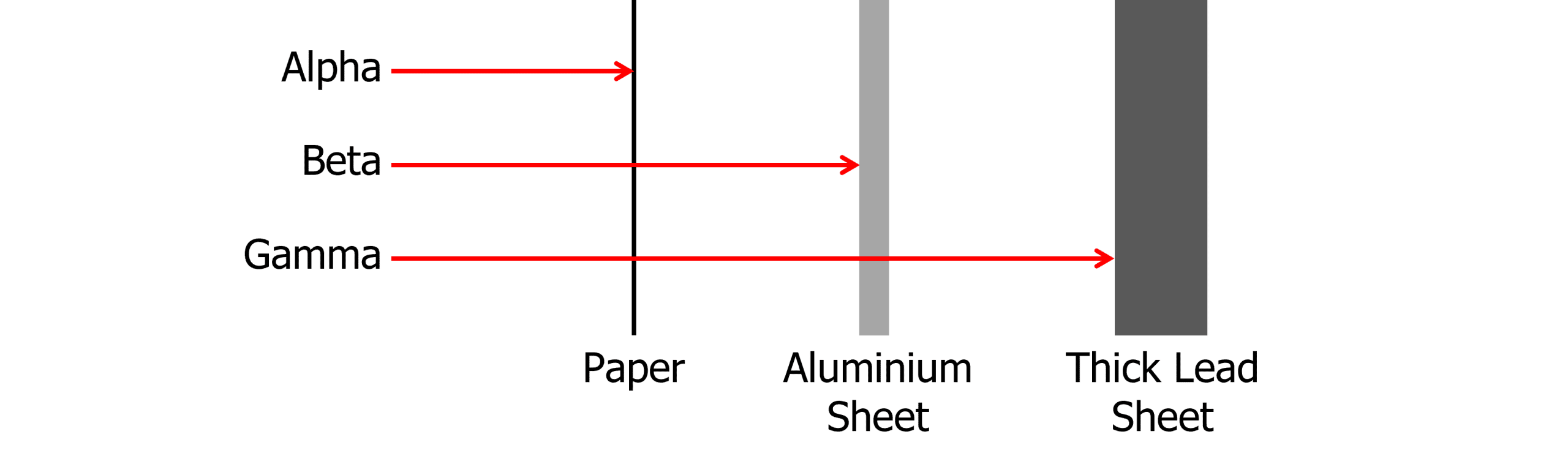
- Not very penetrating (because of it’s relatively large size)
- Short range in air (about 5 \text{ cm})
- Can easily be stopped by a thin piece of paper
Often an unstable atom will emit an alpha particle to reduce its mass in order to become more stable.
Beta Radiation
A single beta particle consists of one electron or one positron. For beta minus decay, an electron is emitted, for beta plus decay, a positron is emitted.

A beta minus particle is emitted when a neutron spontaneously becomes a proton in the nucleus to reduce the number of neutrons and become more stable.
A beta plus particle is formed when a proton spontaneously becomes a neutron in the nucleus to increase the number of neutrons and become more stable.
Some properties of beta radiation include:
- Moderately ionising (charged particles)
- Moderately penetrating
- Range of up to 3 \text{ m} in air
- Stopped by aluminium foil
Gamma Radiation
Gamma radiation is the odd one out. Unlike alpha and beta which are particles, gamma is a high energy electromagnetic wave.
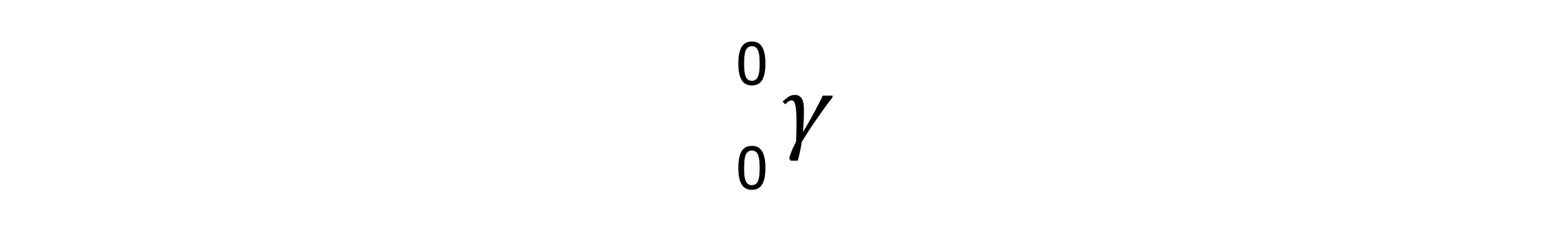
Some properties of gamma radiation include:
- Weakly Ionising
- Highly penetrating
- Not affected by an electric field (because it is not charged and therefore, no electrostatic forces)
Gamma decay occurs to reduce the overall energy of the atom and hence gain stability.
Identification of Radiation Type
You can use your knowledge of the different penetrating powers of radiation to identify the type of radiation being produced by an unknown source. By placing the source of radiation in front of a GM tube and counter, you could start off with a thin piece of paper and place it between the source and the GM tube. If the radiation stops being detected, you know the source must be alpha. Repeat the process with the aluminium and varying thicknesses of lead to identify the penetrating power of the source.
It is important to note that gamma radiation follows the inverse square law. Therefore, for every time the distance from source to detector is doubled, the amount of radiation would quarter:
I \propto \dfrac{1}{x^2}
- I= intensity in becquerels \text{(Bq)}
- x= distance from the source in metres \text{(m)}
Applications of Alpha, Beta and Gamma Radiation
Smoke detectors
Americium-241 is used in smoke detectors. It is a weak alpha source which emits particles within the smoke detector.
The alpha particles cause the ionisation of oxygen and nitrogen within the casing of the smoke detector. When the nitrogen and oxygen are ionised, they conduct electricity, completing a circuit within the smoke detector. While this circuit is complete, no alarm will sound.
However, as smoke enters the detector, the alpha particles are absorbed preventing them from ionising the oxygen and nitrogen. This causes the circuit to be incomplete and the alarm to sound.
The alpha source is used as it cannot escape the smoke detector and has a long half-life so its activity will remain high throughout the lifetime of the smoke detector.
Thickness detectors
Beta radiation is used in machines that make paper, plastic or metal into thin sheets. A source of beta radiation is placed on one side of the sheet and a detector is placed on the other side.
The machine is calibrated to aim for a specific amount of beta radiation detected by the detector. If the detector has a reading of less than this amount, this would indicate too little radiation is passing through the sheet and the sheet is too thick. Therefore the rollers that make the thin sheet squeeze closer together to make the paper thinner. The opposite happens if the sheet is too thin. Too much radiation passes through so the rollers move apart making the paper thicker.
A beta source with a long half-life is optimal as it is not too penetrating that it is not affected by the change in thickness (gamma), but also not too low penetration that it cannot pass through (alpha).
A similar principle can be applied for thicker materials using a gamma source.
Background Radiation
There is a low level of radiation around us at all times. If you were to take a Geiger counter, it would always pick up a low level of activity. This radiation is known as background radiation. Background radiation comes from a variety of sources which can be categorised as either natural or man-made:
Natural sources:
- Cosmic rays from space – high speed protons emitted from the sun produce small amounts of gamma radiation as they enter the earth’s atmosphere.
- Carbon\bold{-14} – all organic matter contains small amounts of the radioactive isotope carbon-14.
- Rocks and soil – small amounts of radioactive isotopes such as uranium are found within rocks and the soil. Uranium naturally decays into radon which is emitted as an alpha source.
- Food and drink – food and drink naturally absorbs radiation from soil and rocks around them. Bananas contain a relatively high amount of potassium-40 but low enough that does not cause a problem.
Man-made sources:
- Medical radiation – radiation from medical scanning and tracers.
- Nuclear waste – very small amounts of radiation is emitted from radioactive waste.
- Nuclear weapons – after a nuclear weapon is used, leftover nuclear residue (fallout) is left in the area. The fallout is an emitter of background radiation.
Required Practical 12
Showing that the intensity of gamma radiation follows the inverse square law.
Doing the experiment:
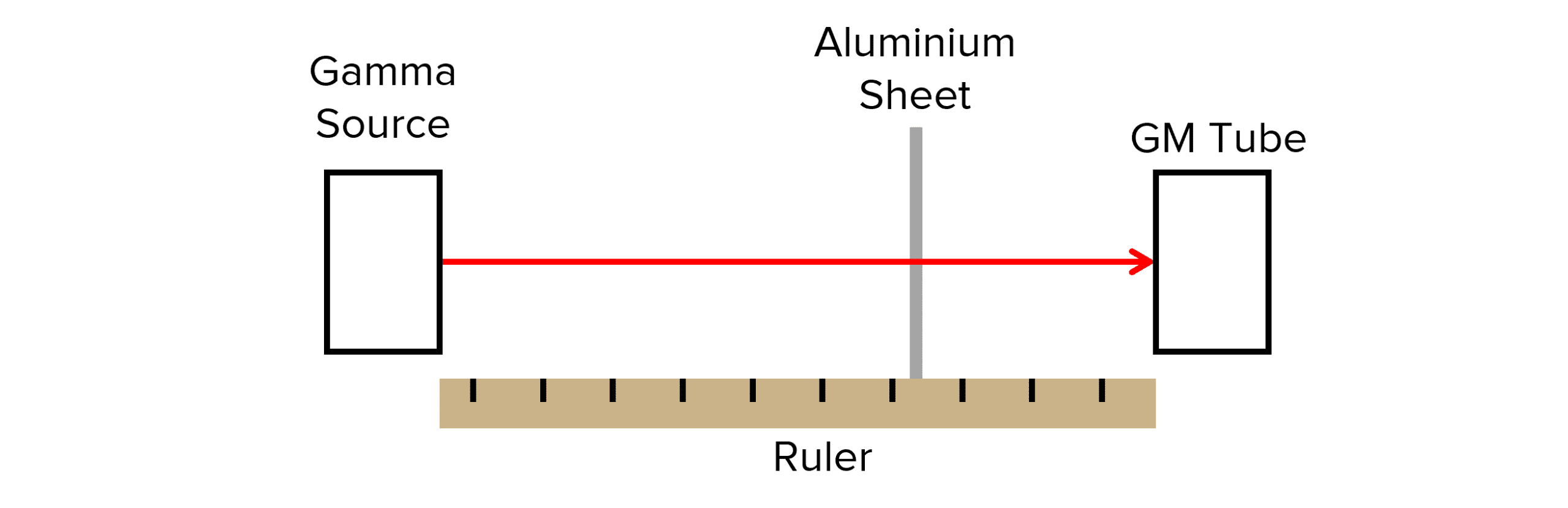
- Set up the experiment as shown in the diagram.
- Measure background radiation using the GM tube when the gamma source is not present. Measure the background radiation for 60 \text{ s} and the mean average of 5 readings should be taken.
- Place the gamma source 5 \text{ cm} away from the GM tube (ensuring the aluminium sheet is between the source and the GM tube) and record the count rate (number of counts per minute).
- Subtract the background radiation reading measures at the start from each of the count rate readings to ensure the count rate is for the radiation produced by the source only.
- Repeat in intervals of 5 \text{ cm}.
- Repeat each distance 5 times then find a mean average count rate for each distance.
Analysing of Results:
Before analysing the results it’s important to discuss the significance of the aluminium sheet. As alpha and beta cannot pass through the aluminium sheet of this thickness, the sheet ensures we only measure the gamma radiation produced by the source.
Plot a graph of count rate on the y-axis and \dfrac{1}{d^2} on the x-axis (where d is distance in metres). If the graph produced is a linear graph through the origin, then the graph shows the count rate is directly proportional to \dfrac{1}{d^2} which is known as the inverse square law.
As this experiment involves the handling of radiation it is vital to consider the following safety precautions:
- Minimise exposure to the gamma source by keeping it in the lead lined box as long as possible.
- Only handle the source with tongs.
- Stand behind the gamma source to avoid exposure to the gamma radiation and stand as far from the source as possible.
Alpha, Beta and Gamma Radiation Example Questions
Question 1: Describe what makes an alpha source suitable to be used in a smoke detector.
[2 marks]
The alpha radiation is highly ionising so it is good at ionising the gases inside the smoke detector. It also is not very penetrating so it is absorbed by smoke.
Question 2: Why would a gamma source not be suitable in detecting the thickness of paper?
[1 mark]
Gamma is highly penetrating and will not be absorbed by paper.
Question 3: Give one natural and one man-made source of background radiation,
[2 marks]
Any one natural source from:
- Cosmic rays
- Carbon-14
- Rocks and soil
- Food and drink
Any one man-made source from:
- Medical radiation
- Nuclear waste
- Nuclear weapons