Rutherford Scattering
Rutherford Scattering Revision
Rutherford Scattering
The model of the atom and the nucleus has changed over time with advances in technology. In this section we look at Rutherford’s alpha scattering experiment and his findings.
Rutherford Scattering
Ernest Rutherford conducted his alpha particle scattering experiment at the beginning of the century. His experiment is shown below:
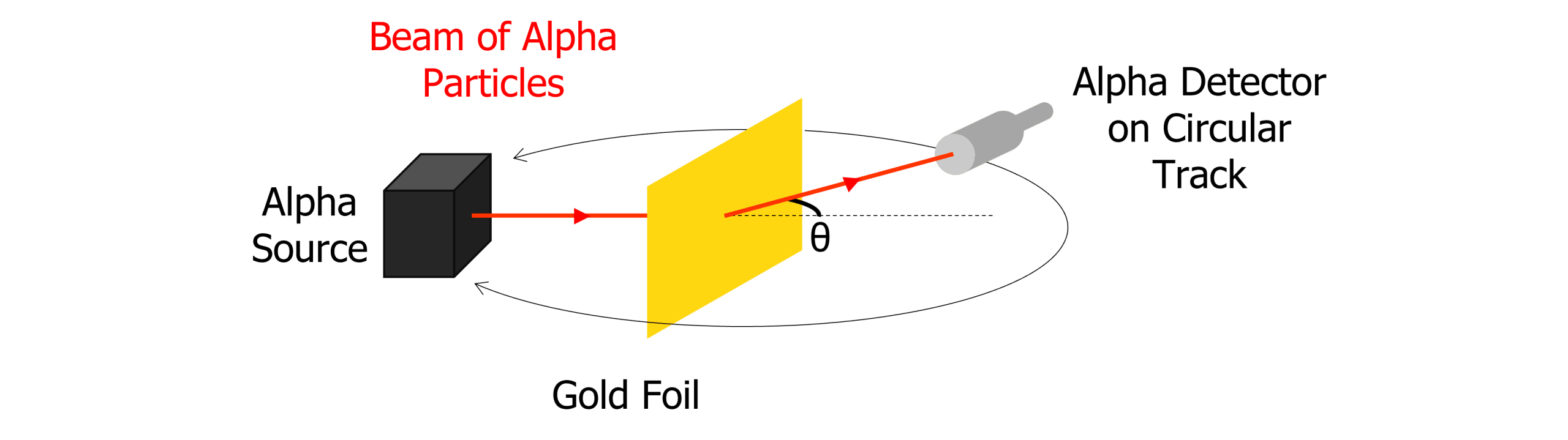
Alpha particles were fired at a sheet of gold foil approximately a micrometre in thickness. The alpha particles (two protons and two neutrons) are positively charged, just like the nucleus of the gold particles in the gold foil.
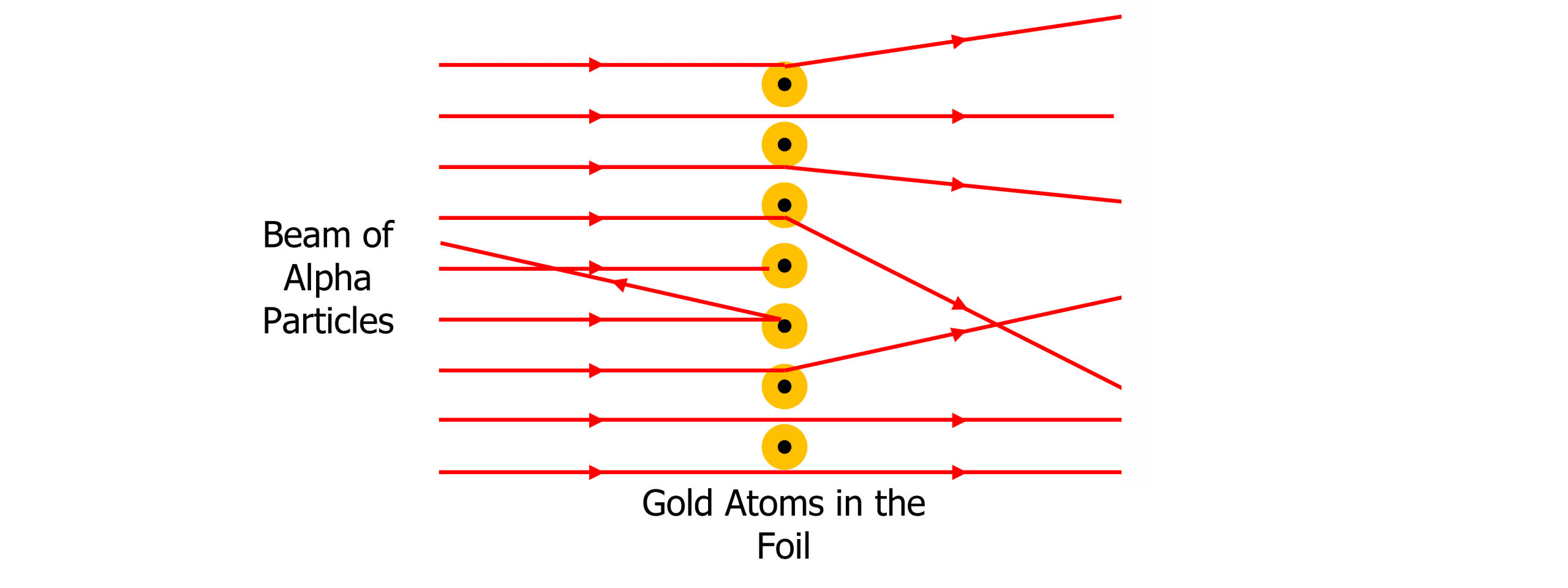
Rutherford found that the majority of particles passed straight through and were detected directly opposite the particle source. However, some alpha particles were slightly deflected at small angles (less than ten degrees) whilst a very small number were reflected back towards the source. Rutherford concluded:
- The majority of the atom is empty space – supported by the fact that most particles passed straight through.
- The nucleus is positively charged – supported by the detection of some alpha particles being deflected at small angles as like charges repel.
- The nucleus is tiny but dense (all the mass of the atom is concentrated in the nucleus) – supported by the reflection of only a tiny number of alpha particles.
Further Models of the Atom
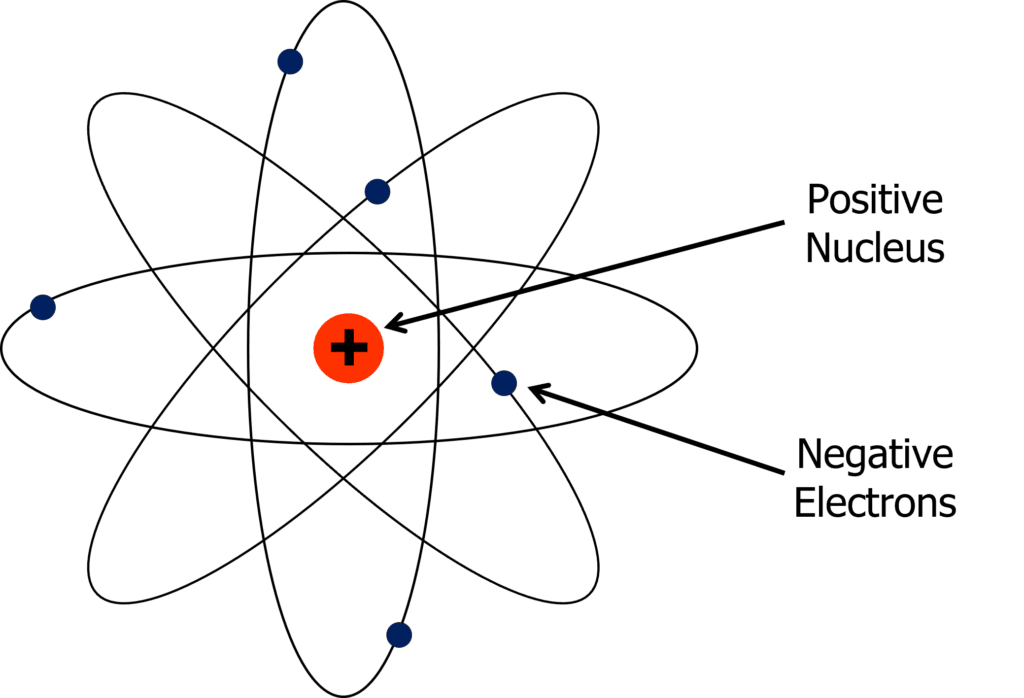

Neils Bohr proposed the idea that the nucleus was made up of positive protons, and the electrons orbited the nucleus in energy levels. This model is shown on the right.
Later in the 20^{th} century, James Chadwick discovered the neutron, giving us our current accepted model of the atom.
Rutherford Scattering Example Questions
Question 1: What evidence did the alpha scattering experiment give for the nucleus being tiny?
[1 mark]
Only a small number of particles were reflected back towards the alpha source.
Question 2: What evidence did the alpha scattering experiment give for the nucleus being positively charged?
[2 marks]
Some of the alpha particles were deflected at small angles.
This was due to the electrostatic repulsion between two positive particles.
Question 3: What did Niels Bohr add to the discovery of the nucleus?
[2 marks]
Niels Bohr experimentally discovered the positive proton and that the electrons existed in discrete energy levels.









