Commercial Applications of Electrochemical Cells
Commercial Applications of Electrochemical Cells Revision
Commercial Applications of Electrochemical Cells
Electrochemical cells can be used as a commercial source of electrical energy. These cells can either be non-rechargeable, rechargeable or fuel cells.
Lithium Ion Cells
Lithium cells, also known as \text{Li}-ion cells or \text{Li}-ion batteries are one of, if not the, most commonly used electrochemical cells. Almost all handheld electronics (i.e. phones, tablets, portable games deceives etc.) contain a \text{Li}-ion battery. These cells are also used for larger applications, such as electric vehicles.
\text{Li}-ion cells typically consist of a solid lithium oxide cathode such as lithium cobalt oxide \left(\text{LiCoO}_2\right) and a \text{Li}^+ containing anode, such as graphite with \text{Li}^+ ions held between the graphene layers.
The overall reaction in \text{Li}-ion cells is \text{Li} + \text{CoO}_2\rarr\text{LiCoO}_2.
In \text{Li}-ion cells, different reactions take place at each electrode.
At the positive electrode: \text{Li}^+ + \text{CoO}_2 + \text{e}^-\rarr \text{Li}^+\text{[CoO}_2\text{]}^–
At the negative electrode: \text{Li}\rarr\text{Li}^+ +\text{e}^-
The conventional cell representation for a lithium cell is:
\text{Li}_{\text{(s)}}\vert\text{Li}^+_{\text{(aq)}}\Vert\text{Li}^+_{\text{(aq)}}, \text{CoO}_{2\text{(aq)}} \vert\text{LiCoO}_{2\text{(s)}} \vert\text{Pt}
Rechargeable Cells
On reason for the widespread use of Li-ion batteries is their rechargeable nature. Rechargeable cells are cells in which the anode and cathode reactions are both rechargeable. This means that, unlike in non-rechargeable batteries like acid-alkali or lead-acid batteries, the reactants in rechargeable batteries are not permanently used up. When undergoing recharging, the anode and cathode reactions are driven in the reverse direction, regenerating the original reactants.
To recharge a cell, a current is applied. This dives electrons from the cathode to the anode, feeding the reverse reactions taking place at these electrodes.
Rechargeable cells have the advantage of producing less waste. They are able to be used for much longer than non-rechargeable batteries meaning fewer batteries (and so less chemical products) go to landfill. However the materials used in rechargeable batteries often require large scale mining operations which can off set the environmental benefits of less waste.
Fuel Cells
Fuel cells are a special application of electrochemistry. Fuel cells use the chemical energy from the reaction of a fuel such as hydrogen to create a voltage and generate an electric current. One common example of a fuel cell is the alkaline hydrogen oxygen fuel cell.
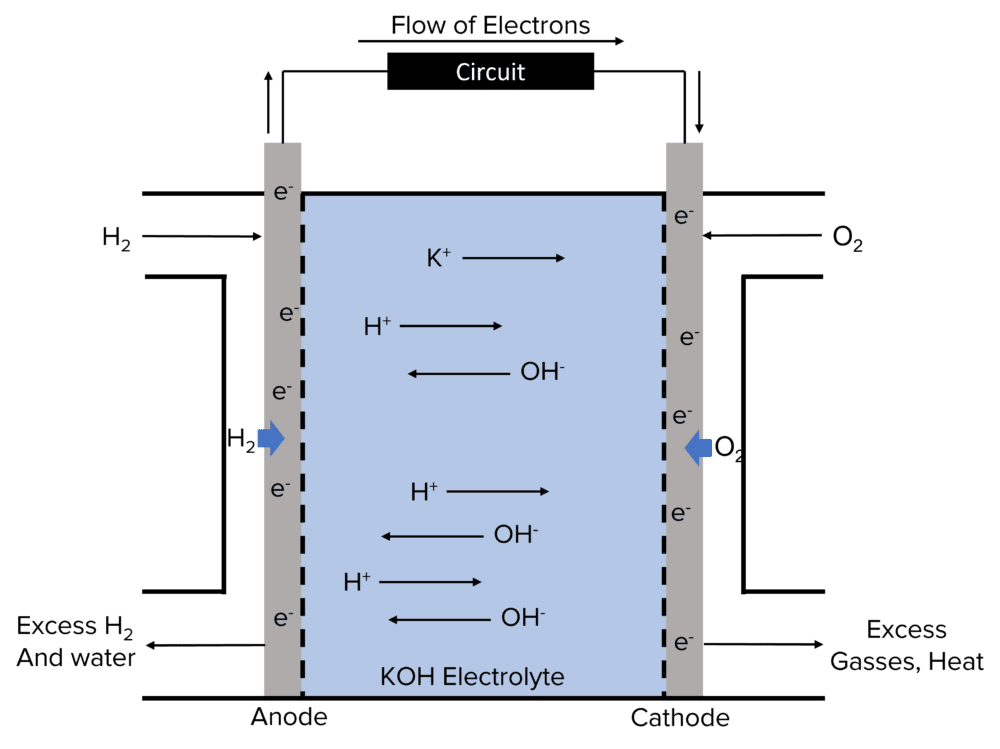
Hydrogen-Oxygen fuel cells are consist of an anode and a cathode, separated by a porous electrolyte of an alkaline charge carrier. Hydrogen is supplied continuously to the anode, and oxygen continuously to the cathode.
At the anode, hydrogen gas is oxidised by \text{OH}^- diffusing across from the cathode to form \text{H}_2\text{O}, \text{H}^+ ions, and electrons. The electrons are taken into the circuit and transferred across to the cathode.
At the cathode, oxygen is reduced by the electrons produced from the anode reaction and water from the air to form \text{OH}^- ions. These \text{OH}^- ion diffuse across the electrolyte to take part in the anode reaction.
In alkaline conditions, the following reactions take place.
At the anode: 2\text{H}_2+4\text{OH}^-\rarr4\text{H}_2\text{O}+4\text{e}^-
At the cathode: \text{O}_2+2\text{H}_2\text{O}+4\text{e}^-\rarr4\text{OH}^-
The overall reaction for the hydrogen fuel cell is 2\text{H}_2 + \text{O}_2\rarr2\text{H}_2\text{O}.
Fuel cells do not use standard conditions because the rate would be too slow to produce enough current. Therefore, higher temperatures and higher pressures are used to increase the rate.
| Advantages of Fuel Cells | Disadvantages of Fuel Cells |
|
|
Commercial Applications of Electrochemical Cells Example Questions
Question 1: Give the half equation for the reaction taking place at the negative electrode of a lithium ion cell.
[1 mark]
\text{Li}\rarr\text{Li}^+ +\text{e}^-
Question 2: Give two advantages of hydrogen fuel cells.
[2 marks]
- They release far less pollution and CO2 when compared to petrol or diesel.
- They also have greater efficiency than petrol or diesel.
Question 3: Give the conventional cell diagram for a lithium cell.
[3 makrs]
\text{Li}\vert\text{Li}^+\Vert\text{Li}^+, \text{CoO}_2 \vert \text{LiCoO}_2 \vert \text{Pt}
Question 4: Give the overall reaction of the hydrogen fuel cell.
[1 mark]
2\text{H}_2 + \text{O}_2\rarr2\text{H}_2\text{O}
You May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.