Conservation of Energy
Conservation of Energy Revision
Conservation of Energy
Energy can be transferred, stored or dissipated (wasted).
But energy cannot be created or destroyed.
This means that in a closed system (where energy cannot leave or enter the system) the net change in energy will always be zero.
Useful and Wasted Energy
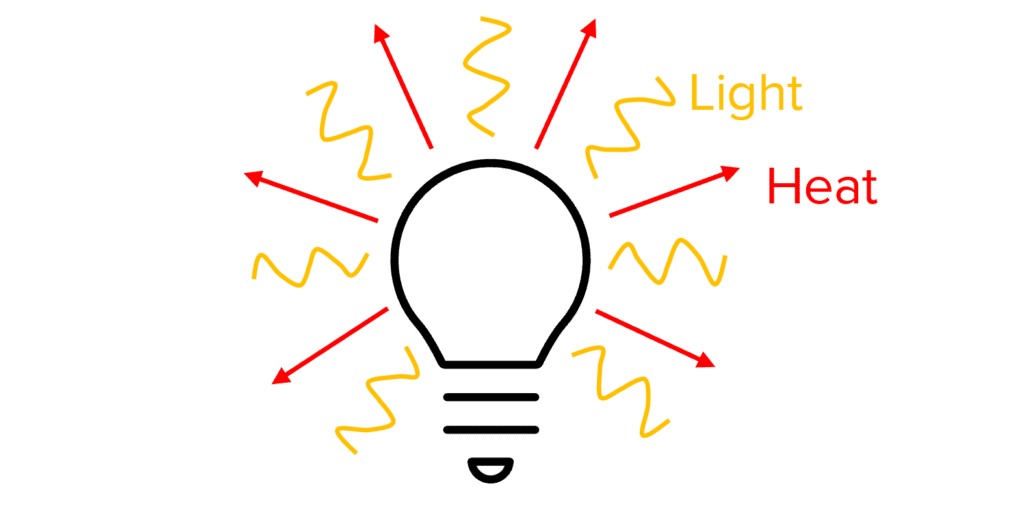
When transferring energy between stores, not all of the energy will be transferred to the desired energy store. The energy that transfers to the intended store is considered the useful energy. The energy transferred to other stores is the wasted energy. We say that the wasted energy has been dissipated.
For example, a light bulb produces both heat and light. The amount of energy that is used to produce the light is the useful energy. The energy that transfers to the heat energy store is wasted, because we did not want the lightbulb to get hot.
The useful and wasted energy always add up to the energy input because energy cannot be created or destroyed.
Reducing Wasted Energy
There are a number of ways that unwanted transfers of energy can be reduced. Thermal insulation and lubrication are two important methods for reducing wasted energy.
Thermal Insulation:
Energy is often lost from a system via heat energy transferring to the surroundings. By covering an object in a layer or multiple layers of a material, you can reduce the rate of heat transfer out of the object. This is called insulation. For example, when you get cold, you might put on a coat. The coat stops the heat energy that your body is produced from transferring to the air around you, and so you don’t get cold as fast.
Lubrication:
Energy can also be lost when friction acts between objects that are rubbing together. The amount of energy lost to friction can be reduced by making the surfaces smoother. This is called lubrication. For example, roads are much more slippery when it is icy, this is because ice is smoother than tarmac and so there is less friction between car tyres and the road.
Thermal Conductivity
Some materials make better thermal insulators than others. This is because of a property called thermal conductivity. Thermal conductivity tells you how well a material transfers heat energy through it.
Materials with a low thermal conductivity transfer less heat energy and therefore make good thermal insulators.
Materials with a high thermal conductivity transfer more heat energy and therefore make poor thermal insulators.
For example, if you heat one end of a metal rod, the other end will also get hot. This is because metal has a high thermal conductivity and so the heat is transferred quickly to the metal. However, if you heat one end of a plastic rod, the other end will stay cool because plastic has low thermal conductivity.
Buildings are constructed with materials with a low thermal conductivity in order to insulate the rooms inside. This allows the building to stay warm in cold weather but cool in the summer.
Required Practical
Investigating the Thermal Conductivity of Different Materials
You can test to see how effective materials are as thermal insulators by performing the following experiment.
Doing the experiment
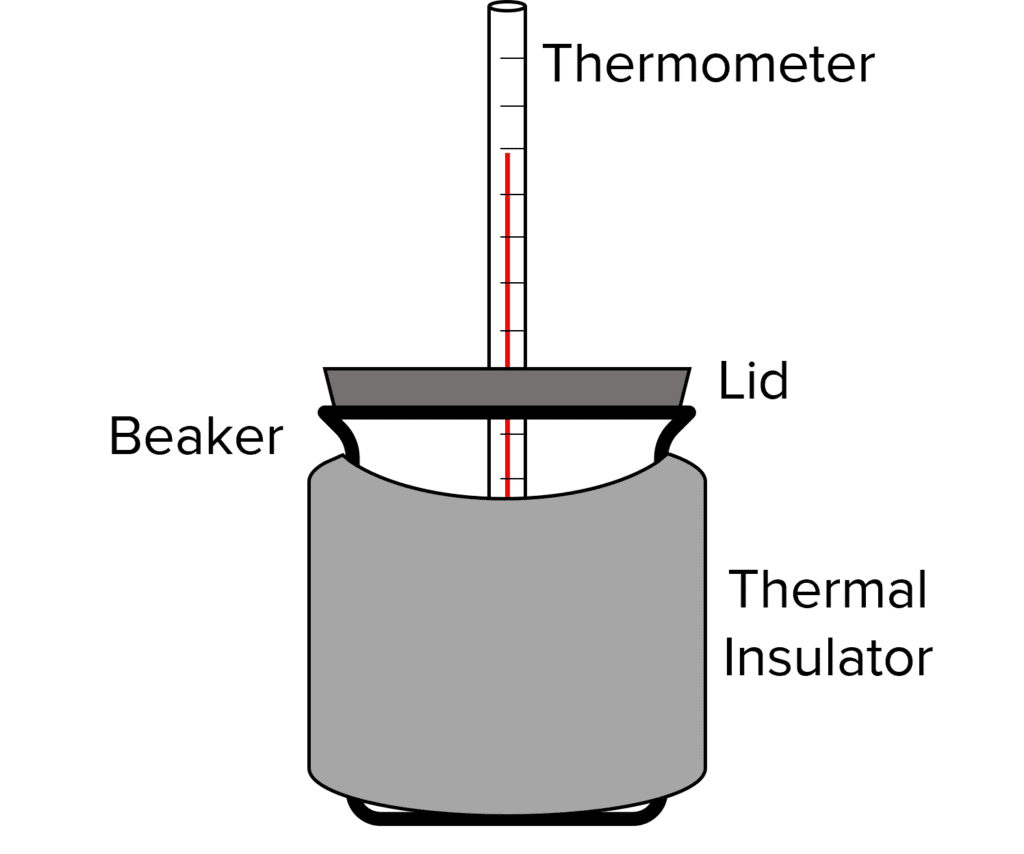
- Boil some water and pour into a room temperature beaker. Place a lid on the beaker.
- Start a stopwatch at the same time as measuring the temperature of the water using a thermometer.
- After 5 \text{ minutes}, measure the temperature of the water again.
- Calculate the decrease in temperature.
- Pour away the water and leave the beaker to cool to room temperature.
- Repeat the experiment, but this time wrap an insulating material, such as bubble wrap, around the beaker.
- Repeat with a range of different materials and different thickness of materials.
The smaller the decrease in temperature, the better the thermal insulator. The bigger the decrease in temperature, the worse the thermal conductor.
Conservation of Energy Example Questions
Question 1: Suggest one method for reducing wasted heat from transferring out of a house.
[1 mark]
Any one from:
- Loft insulation
- Double-glazed windows
- Wall insulation
- Thicker walls
- Use of draught excluders
Question 2: Explain why heat loss is reduced in a bicycle chain and cog after the chain is covered in oil.
[2 marks]
The oil reduces friction between the chain and the cog. Friction produces heat and so less heat is produced. This is called lubrication.
Question 3: Explain why water cools down faster in a metal container than in a plastic container?
[2 marks]
Metal has a higher thermal conductivity than plastic so heat energy is transferred faster through the metal than plastic.





