Potable Water
Potable Water Revision
Potable Water
All living things need water to survive. For human beings, this water must be potable. Potable water can be produced from fresh water using filtration and sterilisation. It can also be produced from sea water using desalination, though this process is much more expensive.
What is Potable Water?
Water is essential to life on earth. All living organisms rely on water for their survival and need this water to be safe to consume. All water contains at least small quantities of contaminants in the form of dissolved salts and naturally occurring bacteria. In small quantities, contaminants do not pose a risk to the health of the consumer. Above a certain concentration however, contaminants will cause sickness. For example, water with a high concentration of bacteria can spread diseases when consumed.
Water that contains concentrations of salts or bacteria that are too low to cause harm is known as potable water. Potable water is not to be confused with pure water (in the chemical sense). Chemically pure water contains no other molecules other than \text{H}_2\text{O} whereas potable water, though safe to drink, is likely contain small quantities of dissolved matter and micro-organisms.
Water can be non-potable for a number of reasons. Sewage water is non-potable because it contains a high level of bacteria that would lead to sickness if consumed where as sea water is non-potable due to its high salt concentration.
Fresh Water Sources of Potable Water
In the UK, the majority of our freshwater comes from the filtration and sterilization of freshwater sources. The rainy climate of the UK provides a source of water with a low concentration of dissolved salts. This rain water falls to the earth, where it collects to form surface water (e.g. rivers and lakes) and ground water (water that is stored underground).
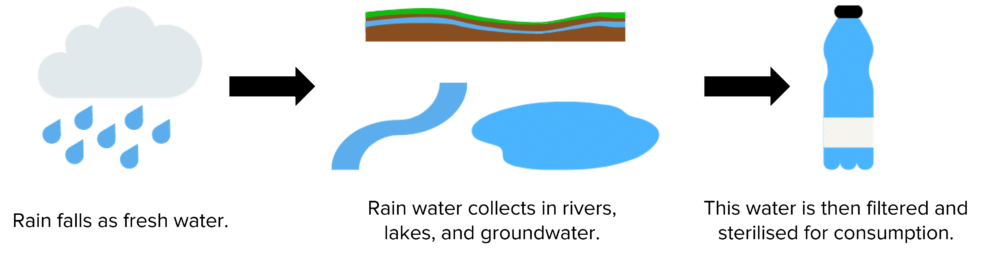
Which sources of water are used to produce potable water will depend on the area of the country and the time of year. For example, in the south of the country where temperatures are highest, surface water often dries out in the summer. During this period, water is taken from ground water sources like aquifers. In the north where temperatures are lower and conditions are wetter, water often comes from sources such as reservoirs and lakes.
Treating Fresh Water
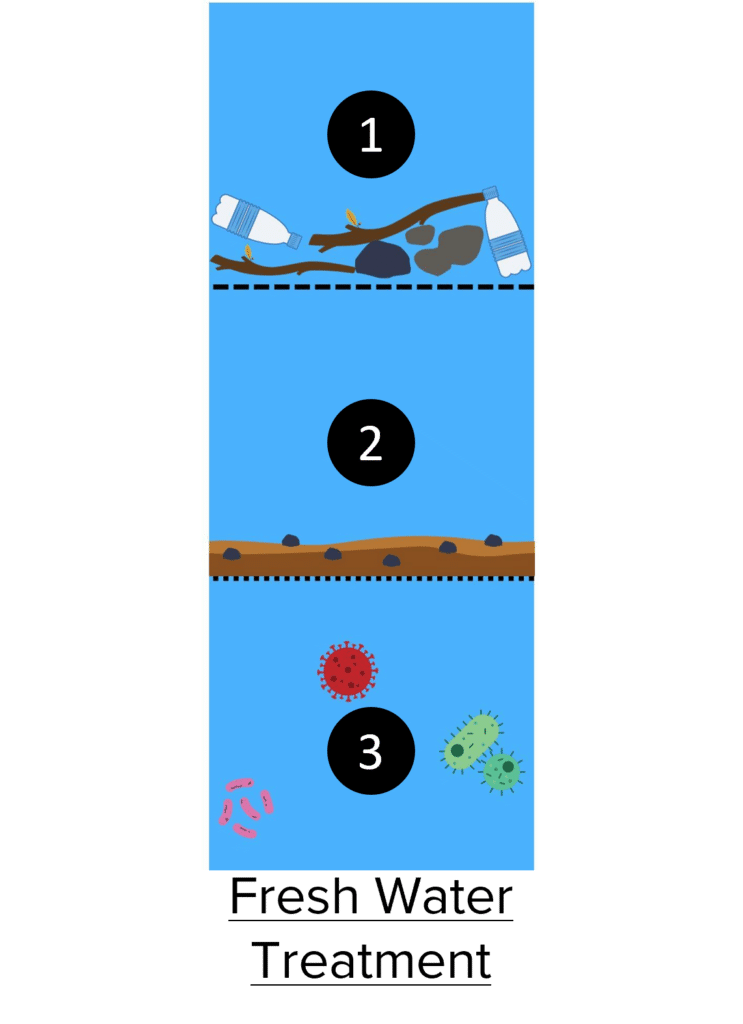
Though fresh water does not contain high concentrations of salts, it must still be treated before it becomes potable. This treatment is typically done in three stages, two filtration steps and then a sterilisation step.
- Filtration of Large Contaminants: In this stage, water is passed through a large mesh filter to remove larger solid contaminants. This step will remove objects like sticks and rocks as well as waste pollution like plastics and refuse.
- Filtration of Sediment: This stage uses a finer mesh called sand and gravel beds to remove sediments such as soils, sand, and gravel.
- Sterilisation: Finally, the water is treated to remove bacteria and other micro-organisms. This is done by treating the water with chemicals such as chlorine or ozone and by exposing it to ultraviolet. This kills the vast majority of micro-organisms present, preventing sickness.
Desalination
The UK has a fairly rainy climate and this give us access to a lot of fresh water. In some countries however, there is significantly less rain and so less access to fresh water. In these circumstances, countries must turn to the sea water to meet their needs.
Sea water is non-potable without treatment due to its high salt concentration. To convert sea water into potable water it is necessary to remove the salt using a process known as desalination. This can be done using one of two methods, distillation or reverse osmosis.
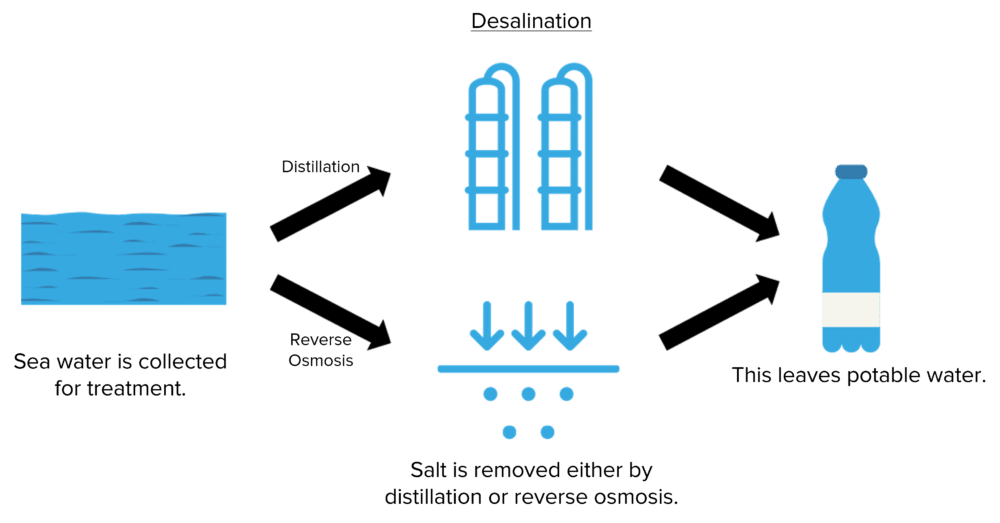
Distillation
In the distillation method, sea water is boiled to produce steam. The steam is piped away leaving only the now solid salt.
The steam is then allowed to condense back into water in another vessel. This water will have had it’s salt content, as well as any solid contaminants, removed. The water can then be further treated to remove any remaining contaminants, leaving only potable water.
Reverse Osmosis
In reverse osmosis, salt water is passed through a permeable membrane. This membrane is designed so that only water molecules can pass through it. Any larger molecules or ions are unable to pass through the membrane and so are left behind when water is passed through. This leaves potable water.
Both reverse osmosis and distillation require large amounts of energy. This makes desalinisation impractical for use on a large scale due to its sizeable cost.
Required Practical
Producing Potable Water
By carrying out a series of experiments, it is possible to produce a sample of clean potable water in the laboratory. To do this, we must first ensure that the water has a neutral \text{pH}. We then need to determine what containments are in the water. Finally, we must distil the water to remove these contaminants. We can therefore break the process down into three steps; titration, chemical tests, and distillation.
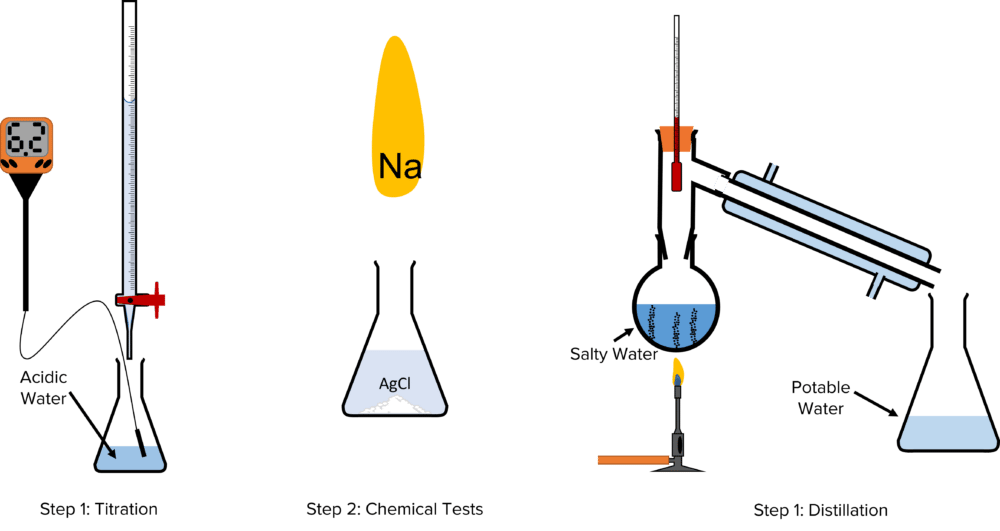
Step 1: Titration
- For water to be safe to drink it must have a neutral \text{pH}.
- Using a digital \text{pH} meter, measure the \text{pH} of the sample of water. (Note a digital \text{pH} meter should be used to avoid contaminating the sample).
- If the water has an acidic or basic \text{pH}, use a titration to neutralise it.
Step 2: Chemical Tests
- Neutralising the water will likely have created a salt. The presence of a salt can be tested for using simple chemical tests.
- Pipette a small sample of the water into a test tube.
- Dip a splint into the test tube sample and then place the damp end into flame of a splint. If the flame turns yellow, sodium is present in the water.
- Add a solution of aqueous silver nitrate to the test tube sample. If a white precipitate, chlorine is present.
- These two tests will indicate the presence of salt in the original sample.
Step 3: Distillation
- Finally, this salt must be removed to produce a sample of potable water.
- The sample of water should be transferred to a round bottomed flask and connected to a condenser. (Remember to ensure that the water flowing into the the condense enters at the bottom).
- The sample should be heated using a Bunsen Burner and brought to the boil.
- Collect the liquid water that is produced in the condenser in a conical flask.
- When all of the water has been distilled solid salt should remain in the condenser, with a sample of potable water collected in the conical flask.
Potable Water Example Questions
Question 1: State the difference between chemically pure water and potable water.
[1 mark]
- Chemically pure water contains \text{H}_2\text{O} molecules only.
- Potable water can contain dissolved salts and naturally occurring bacteria.
Question 2: State one source of fresh water.
[1 mark]
Any one from:
- Rivers
- Lakes
- Ground Water
(Allow reservoirs)
Question 3: State why desalination is used less often despite the abundance of sea water.
[2 marks]
- Desalination requires a lot energy.
- This means that is an expensive process to run.