Fission and Fusion
Fission and Fusion Revision
Fission and Fusion
Nuclear fission and nuclear fusion are both processes that happen at the nuclear level in which lots of energy is produced. If harnessed correctly, these processes have endless possibilities for their use.
Nuclear Fission
Nuclear fission involves the splitting of a larger nucleus into multiple smaller nuclei. The process produces several products but also huge amounts of energy. The process of a fission reaction can be seen below:
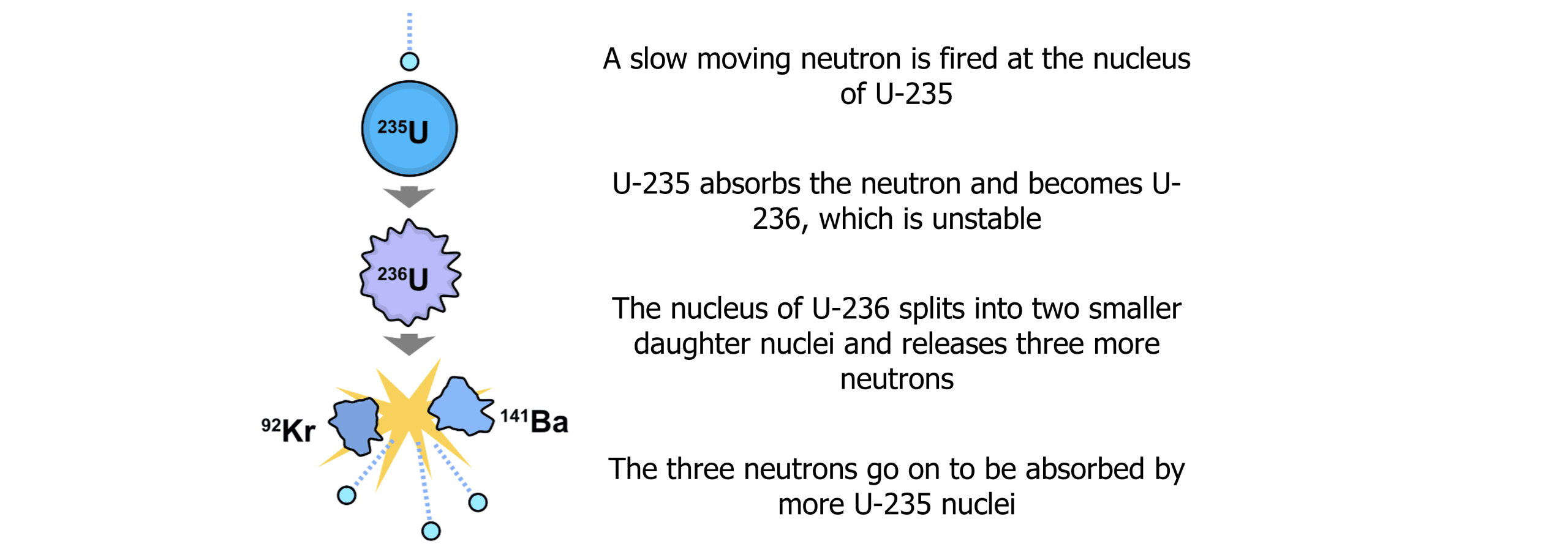
Several neutrons produced from each fission reaction may then cause another fission reaction to occur if they collide with another U-235 nuclei, triggering a chain reaction. This chain reaction is controlled, and the energy harnessed in a nuclear reactor. The number of neutrons produced is dependent on the element undergoing fission.
It is important to note that the neutrons must be slow moving, because a fast moving neutron will rebound off of the U-235 nucleus without fission occurring.
All fission reactions have a critical mass. This is the minimum mass of fuel (U-235) required for the reaction to be self-sustaining. Lower than the critical mass and the reaction will slowly come to a stop. Beyond the critical mass, the reaction becomes uncontrollable.
Nuclear Fusion
Nuclear fusion is the process of two smaller nuclei fusing to form one larger nucleus, giving off huge amounts of energy in the process.
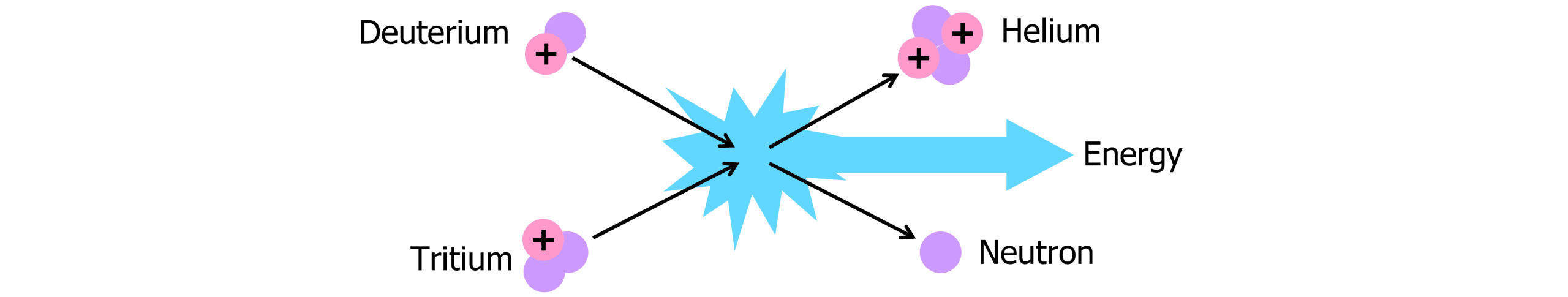
Tritium and deuterium undergo nuclear fusion in the sun in a process which creates massive amounts of energy:
In this process, deuterium and tritium must have very large kinetic energies before they collide. If they have too little kinetic energy, the nuclei will never overcome the force of electrostatic repulsion. Once the nuclei get close enough, the strong nuclear force takes over, fusing the nuclei to form Helium and an emitted neutron.
Although the process produces massive amounts of energy, it also requires huge amounts of energy in the form of heat and pressure which proves to be expensive to produce. Because of the need for extreme temperatures and pressure, the correct conditions are hard to maintain for the amount of time needed.
Energy Calculations
The energy produced as a result of nuclear fission or fusion can be calculated using the equation:
E=\Delta mc^2
- E= energy in joules \text{(J)}
- \Delta m = the mass defect in kilograms \text{(kg)}
- c= the speed of light (=3 \times 10^8 \text{ ms}^{-1})
The energy released is due to a difference in mass between the daughter and parent nuclei.
Binding Energy
When comparing the stability of nuclei, it is useful to compare the binding energy per nucleon. The higher the binding energy per nucleon, the more stable the nuclei because more input energy is needed to split the nucleus into its components.
This can be represented in a graph as shown below:
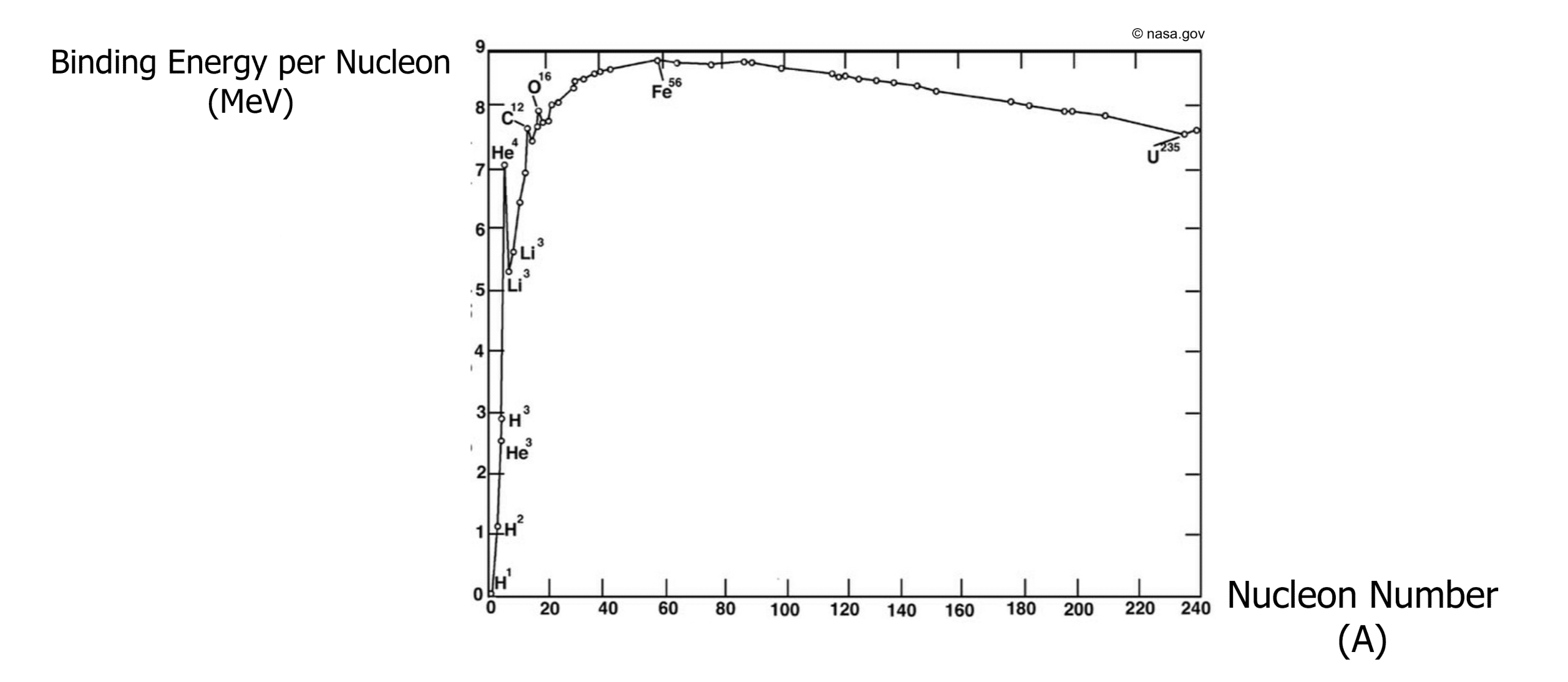
From the graph we can see that iron-56 is the most stable with the highest binding energy per nucleon (8.9 \text{ MeV}).
At low nucleon numbers, nuclei are least stable. Hydrogen and Helium have very small electrostatic forces holding the nuclei in place and therefore are unstable.
Higher values of nucleon number such as U-235, the binding energy begins to decrease as the nucleus gets too large to remain stable.
Nuclear Reactors
A nuclear reactor uses fission reactions to produce large amounts of thermal energy. The thermal energy is then used to heat water into high pressure steam which is pumped through a turbine to turn a generator and produce electricity.
Within the nuclear reactor, there are three distinct components:
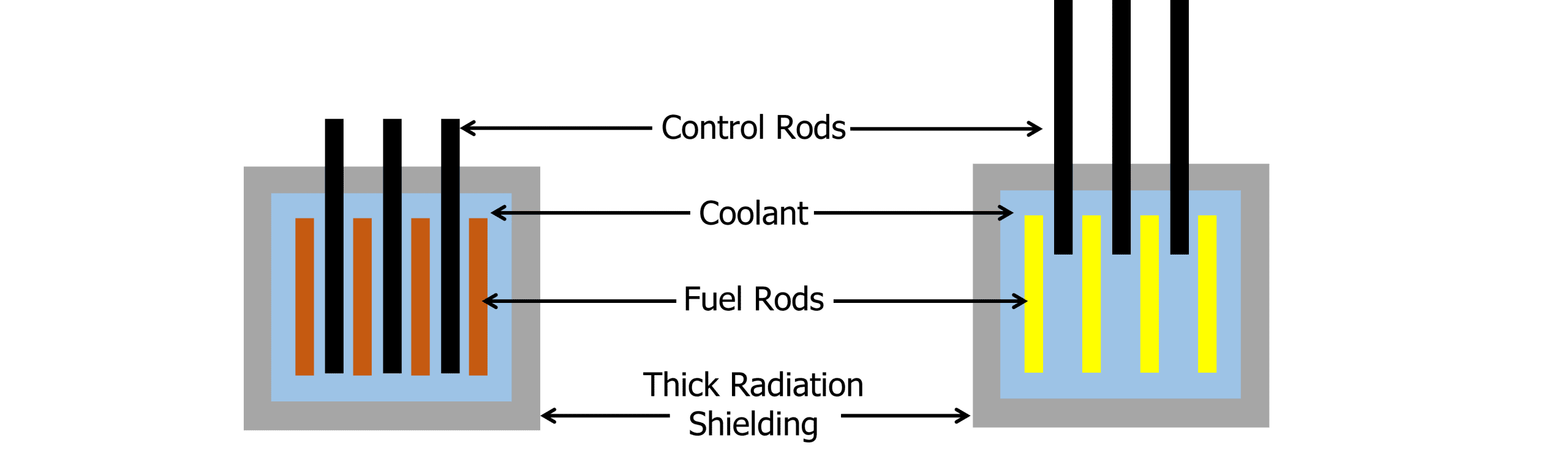
The reactor is surrounded by a thick insulator that keeps heat inside but also minimises radiation leaks through the walls of the reactor.
The fuel rods, usually made of uranium, contain the radioactive nuclei used in the reaction.
The control rods can be lowered and lifted to control the rate of the reaction. The control rods absorb neutrons. They are lowered to absorb neutrons and slow the rate of reaction and lifted to allow more of the chain reaction to occur.
The coolant absorbs the thermal energy and is pumped to a boiler where water is heated into high pressure steam.
Safety in Nuclear Reactors
Nuclear reactors are generally perceived as being dangerous to the public. However, this is mostly through lack of knowledge.
Nuclear power plants all produce radioactive waste. Low level waste includes materials that are lightly contaminated such as clothing etc. This waste is encased in concrete, stored underground and only disposed of after a few years when the radioactivity is at a safe level.
Moderate level waste is more radioactive than low level waste and contains the control rods, and lots of the equipment that is used daily over a long period of time. This waste is stored underground but much deeper than low level and is stored in steel lined drums.
High level waste is the most radioactive waste. It includes spent fuel rods and must be handled with care. This waste is placed in cooling ponds to reduce the high temperatures before being stored carefully underground for hundreds of years.
Fission and Fusion Example Questions
Question 1: Why is it difficult to harness nuclear fusion reactions on earth?
[2 marks]
Fusion reactions require extreme temperatures and pressures.
These are expensive and difficult to maintain.
Question 2: What is the role of control rods in a nuclear fission reactor?
[2 marks]
To maintain a steady rate of reaction. They slow a reaction down when lowered into the reactor by absorbing neutrons before they cause a chain reaction.
Question 3: Explain how the spent fuel rods would be disposed of in a safe manner.
[2 marks]
Spent fuel rods are high level waste. They are first made to cool from high temperatures in cooling ponds before being stored underground for hundreds of years.
Fission and Fusion Worksheet and Example Questions
Energy, Fission and Fusion Questions
A LevelOfficial MMEYou May Also Like...

MME Learning Portal
Online exams, practice questions and revision videos for every GCSE level 9-1 topic! No fees, no trial period, just totally free access to the UK’s best GCSE maths revision platform.