Enzymes
Enzymes Revision
Enzymes
Enzymes are important proteins that control chemical reactions in all living organisms. Each enzyme is specific to an individual reaction and requires certain conditions to function efficiently.
What are Enzymes?
All living organisms perform a range of different chemical reactions all the time which need to be tightly controlled. The sum of all these reactions in an organism or cell is called metabolism. The rate of a reaction can usually be controlled by changing the temperature but cells of the body will get damaged if the temperature is raised too high. Therefore, living organisms use large proteins called enzymes to speed up reactions.
Enzymes are known as biological catalysts which means they increase the rate of biological reactions without being changed or used up.
Enzymes are made up of long chains of amino acids that are folded into specific shapes.
The ‘Lock and Key’ Model
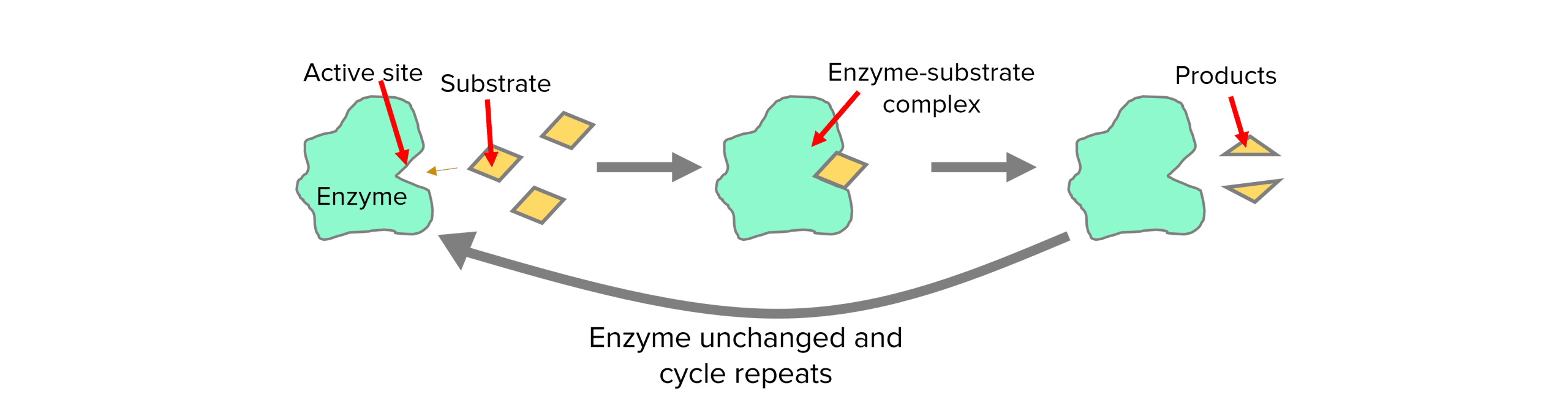
All enzymes have an active site which is where it can bind to a substrate (substance involved in the reaction).
Enzymes and their active sites have very specific shapes. The specific shape of each active site means only certain substrates can bind to it and form enzyme-substrate complexes. This means each enzyme can only catalyse one specific type of reaction and therefore produce the products.
The ‘lock and key’ model is a simplified version of how enzymes work. It states that the active site of an enzyme fits the substrate perfectly like a lock and a key, they are complementary.
Denaturing of Enzymes
The rates of enzyme-controlled reactions are affected by temperature and pH.
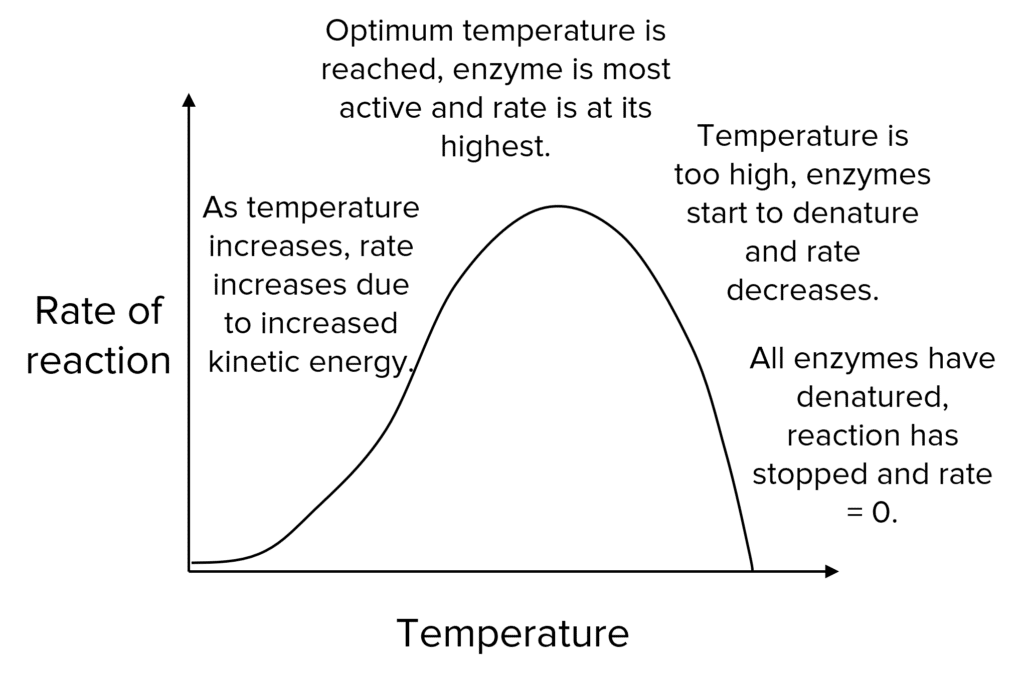
Increasing the temperature will increase the rate of reaction because the enzymes and substrate will have more kinetic energy and so will be more likely to collide and react.
This will only happen up to a point (the optimum temperature). After this, bonds in the enzyme will begin to break, they will lose their specific shape and become denatured.
When the shape of the active site changes, substrates will not be able to bind, enzyme-substrate complexes won’t be able to form and the enzyme will stop catalysing the reaction.
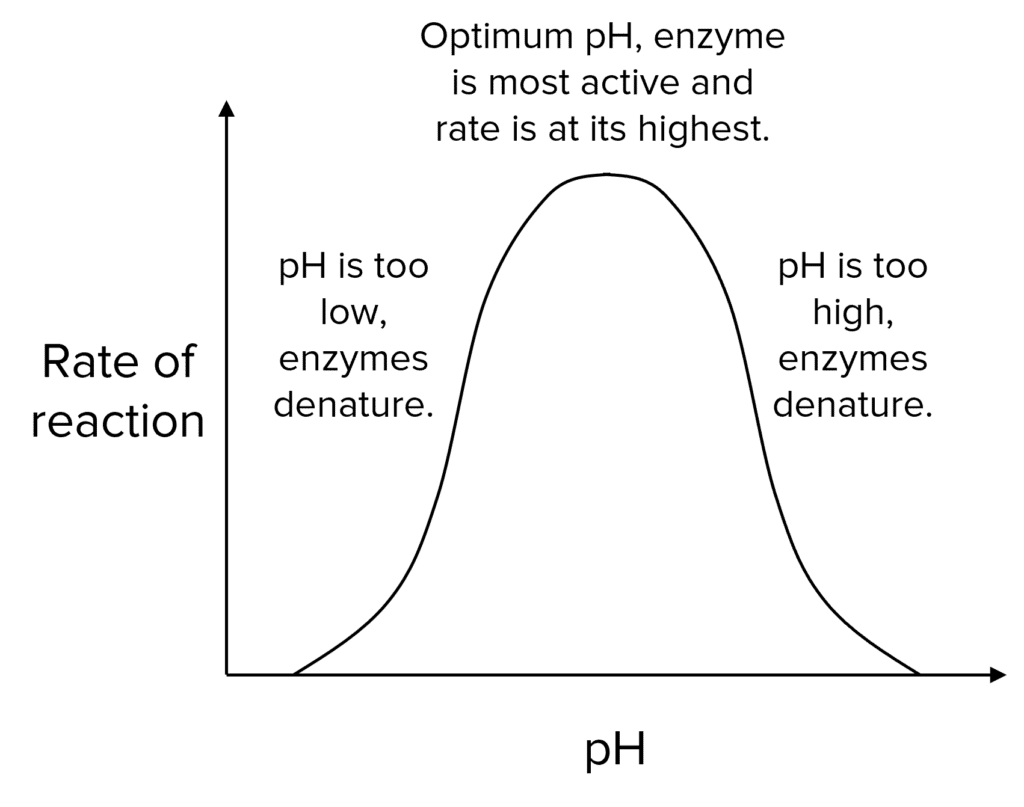
Enzymes also have an optimum pH. If the pH is too high or too low then the bonds in the enzyme will start to break, they will lose their specific shapes and they will become denatured.
If the active site changes shape, substrates will not be able to bind, enzyme-substrate complexes won’t form and so the enzyme will stop catalysing the reaction.
Each type of enzyme will have a different optimum pH. Pepsin is an enzyme that breaks down proteins and works best in the acidic conditions of the stomach.
Required Practical
Investigating the effect of pH on the rate of reaction.
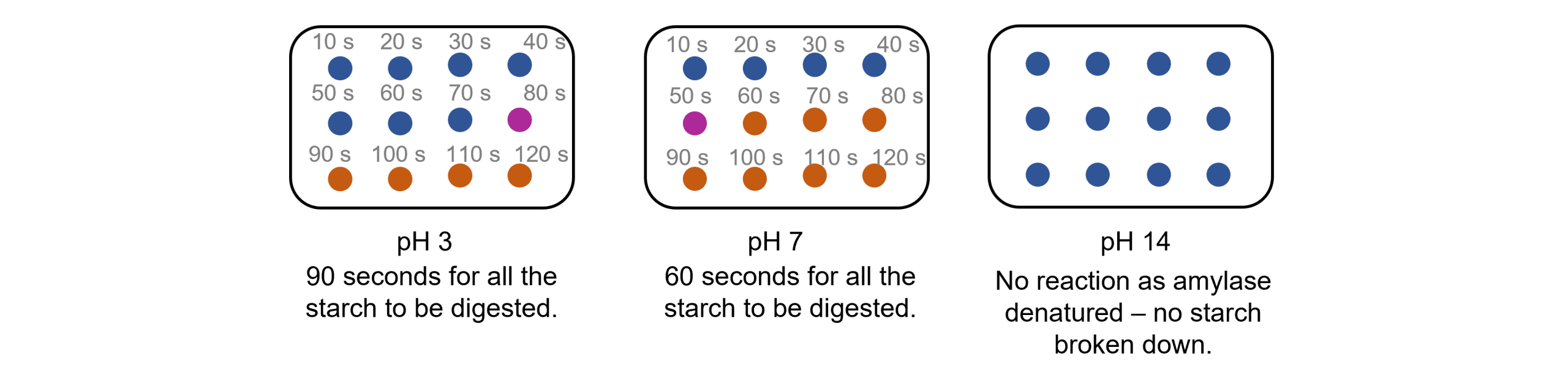
The reaction used for this experiment is the breakdown of starch into maltose by the enzyme amylase. Starch can be easily detected because if added to iodine solution it will turn a blue-black colour. If starch is not present, the iodine solution will remain a brown-orange colour. This makes it very useful for investigating enzymatic reactions.
Doing the experiment
- Using a pipette, prepare spotting tiles by placing a drop of iodine solution in each cavity.
- Prepare a test tube containing 2\text{ cm}^3 of amylase (enzyme) and 1\text{ cm}^3 of a buffer solution with a known pH. Place this in a water bath at 35\text{°C}.
- Prepare another test tube with 2\text{ cm}^3 of starch solution and place it in the water bath.
- Pour the starch into the amylase and buffer solution and start the timer. Make sure to keep the test tubes in the water bath.
- Every 10\text{ seconds} take one drop of the solution and place it on the iodine on the spotting tile. The colour will turn blue-black when starch is present.
- Continue until the colour no longer turns blue-black and remains an orange-brown colour. At this point all the starch has been digested to maltose. The quicker it takes for the spot to remain orange-brown, the faster the rate of reaction.
- Repeat the experiment using buffers of different pH values to find the effect it has on the rate of breakdown of the starch.
It is important to keep the temperature of all the solutions the same, using the water bath, as temperature affects the rate of reaction.
It may also be wise to carry out a control experiment to test if it is actually the amylase that breaks down the starch. This could be done by replacing the amylase with distilled water.
Calculating the rate of reaction
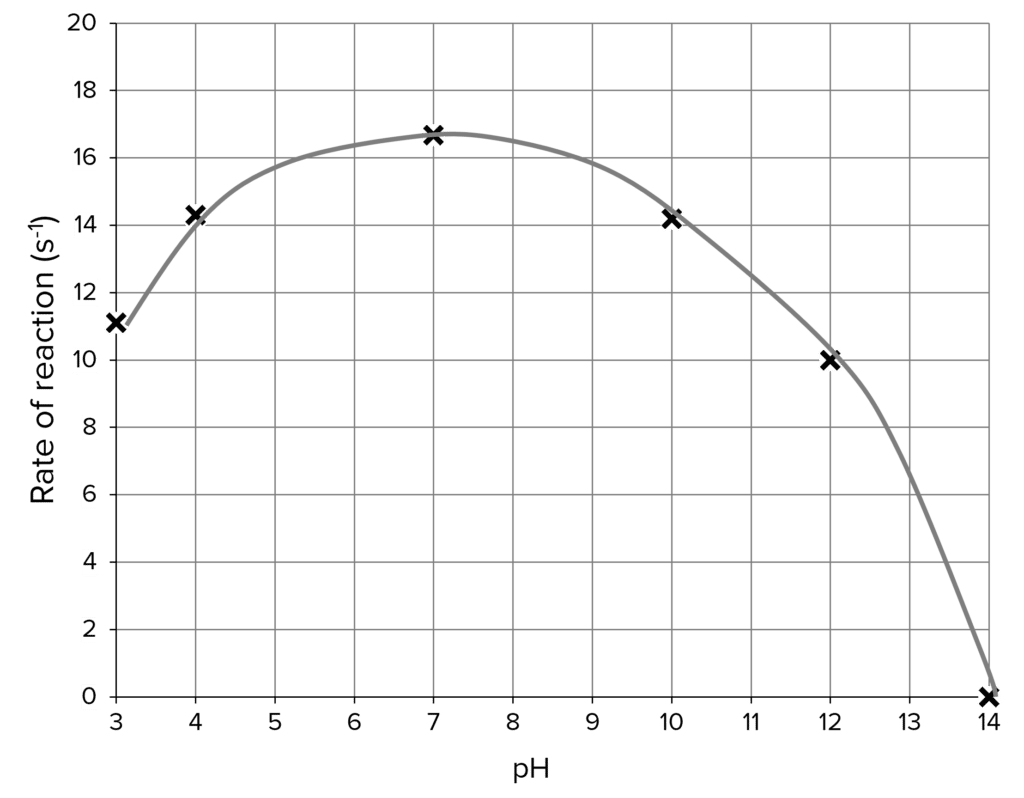
The time it takes for the starch to breakdown and the colour to remain brown-orange is not a measure of the rate of reaction.
Rate is how much something changes over a given time. As the measure of starch breakdown in this experiment is qualitative (changes colour), not quantitative (no numerical values), we can use a constant value for the measure of change.
For this experiment we use 1000:
\text{Rate of reaction (s}^{-1}\text{)} = \dfrac{1000}{\text{Time (s)}}
Now you can plot a graph to show how pH affects the breakdown of starch by amylase.
Enzymes Example Questions
Question 1: Explain the ‘lock and key’ model for enzyme activity.
[3 marks]
The lock and key model states that the active site of an enzyme fits a certain type of substrate perfectly (they are complementary).
This means they can bind together and form enzyme-substrate complexes.
Therefore enzymes can only catalyse specific reactions.
Question 2: Why do enzymatic reactions stop working at high temperatures?
[4 marks]
Bonds within the enzyme will begin to break.
Enzymes will become denatured (lose their specific shape).
Changes to the shape of the active site mean substrates cannot bind (enzyme-substrate complexes cannot form).
Enzymes cannot catalyse the reaction.
Question 3: A student is carrying out an experiment to investigate the effect of temperature on the breakdown of starch to maltose using amylase. They carry out the investigation with the solutions at different temperatures using water baths. They take samples every 10\text{ seconds} and place them on a spotting tile filled with iodine solution.
Below is the results for 50\text{°C}.
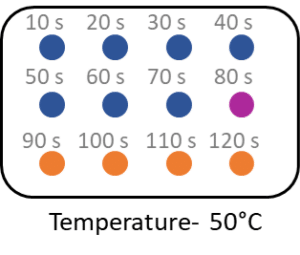
Calculate the rate of reaction at 50\text{°C}.
[2 marks]
\text{Rate of reaction} = \dfrac{1000}{90}
\text{Rate of reaction} = 11.11 \text{ s}^{-1}






